Korean Circ J.
2011 May;41(5):241-247. 10.4070/kcj.2011.41.5.241.
Effect of a Dual Drug-Coated Stent With Abciximab and Alpha-Lipoic Acid in a Porcine Coronary Restenosis Model
- Affiliations
-
- 1The Heart Research Center of Chonnam National University Hospital Designated by Korea Ministry of Health and Welfare, Gwangju, Korea. myungho@chollian.net
- 2Center for Functional Nano Fine Chemicals & School of Applied Chemical Engineering, Chonnam National University, Gwangju, Korea.
- KMID: 2297930
- DOI: http://doi.org/10.4070/kcj.2011.41.5.241
Abstract
- BACKGROUND AND OBJECTIVES
The aim of this study was to examine the anti-proliferative and anti-inflammatory effects of a stent coated with abciximab and alpha-lipoic acid (ALA) in a porcine coronary overstretch restenosis model.
MATERIALS AND METHODS
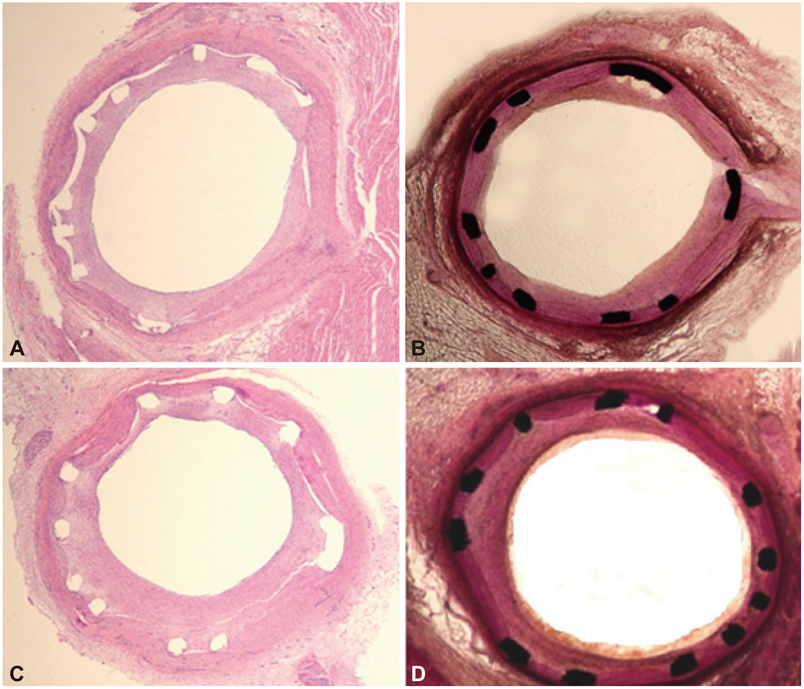
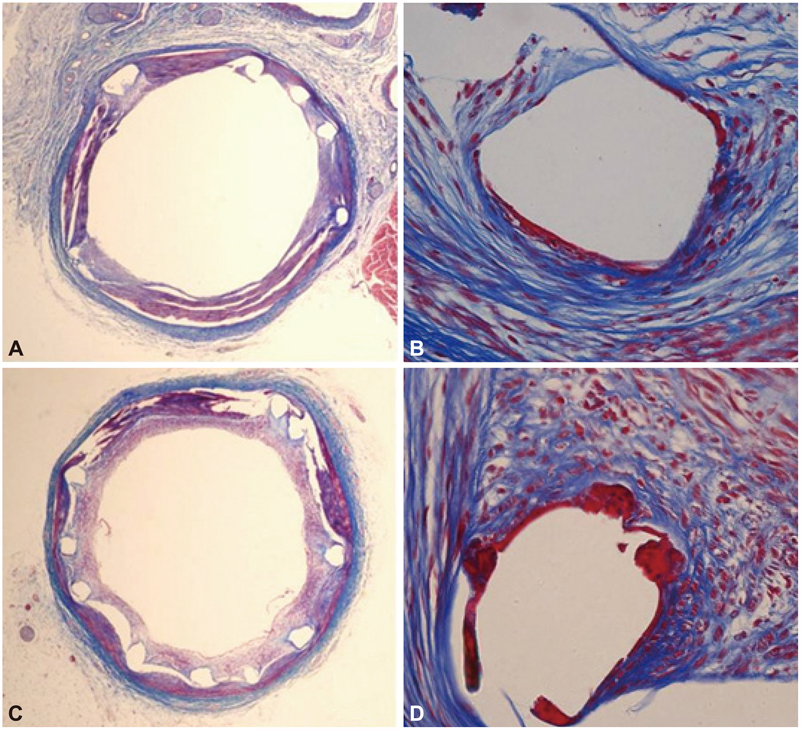
A total of 10 pigs were randomized into two groups (10 pigs, 10 coronaries in each group) in which the coronary arteries were stented with a dual-coated stent and a bare metal stent (control) by randomization. Stents were deployed with oversizing (stent/artery ratio 1.3 : 1) in the porcine coronary arteries, and histopathology was assessed 28 days after stenting.
RESULTS
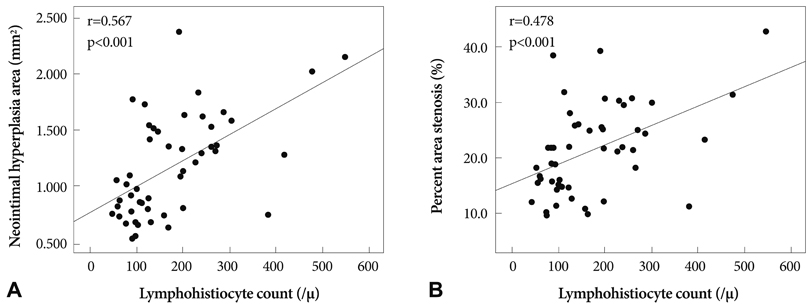
There was no significant difference in the injury score between the two groups. In the neointima, the lymphohistiocyte count was significantly lower in dual-coat stent group compared with the control stent group (120+/-85 cells vs. 159+/-80 cells, p=0.048). There was no significant difference in the fibrin score between the two groups (0.16+/-0.34 in the dual-coated stent group vs. 0.25+/-0.48 in the control stent group, p=0.446). The neointima area was not significantly different between both groups (1.55+/-0.8 mm2 in dual-coated stent group vs. 1.40+/-0.86 mm2 in the control stent group, p=0.447).
CONCLUSION
Although the dual-coated stent with abciximab and ALA showed no significant difference in inhibition of neointimal hyperplasia when compared with the bare metal stent, it was associated with a reduced inflammatory reaction when compared with the control stent in a porcine coronary restenosis model.
MeSH Terms
-
Antibodies, Monoclonal
Antioxidants
Coronary Restenosis
Coronary Vessels
Drug-Eluting Stents
Fibrin
Hyperplasia
Immunoglobulin Fab Fragments
Neointima
Platelet Aggregation Inhibitors
Random Allocation
Stents
Swine
Thioctic Acid
Antibodies, Monoclonal
Antioxidants
Fibrin
Immunoglobulin Fab Fragments
Platelet Aggregation Inhibitors
Thioctic Acid
Figure
Cited by 1 articles
-
Mechanical and Histopathological Comparison between Commercialized and Newly Designed Coronary Bare Metal Stents in a Porcine Coronary Restenosis Model
Kyung Seob Lim, In Ho Bae, Jung Ha Kim, Dae Sung Park, Jong Min Kim, Jung Hyun Kim, Doo Sun Sim, Young Joon Hong, Myung Ho Jeong
Chonnam Med J. 2013;49(1):7-13. doi: 10.4068/cmj.2013.49.1.7.
Reference
-
1. Eeckhout E, Wijns W, Meier B, Goy JJ. Indications for coronary stent placement: the European view. Eur Heart J. 1999. 20:1014–1019.2. Faxon DP, Spiro TE, Minor S, et al. Low molecular weight heparin in prevention of restenosis after angioplasty. Results of Enoxaparin Restenosis (ERA) Trial. Circulation. 1994. 90:908–914.3. Hamm CW, Reimers J, Ischinger T, Rupprecht HJ, Berger J, Bleifeld W. A randomized study of coronary angioplasty compared with bypass surgery in patients with symptomatic multivessel coronary disease. German Angioplasty Bypass Surgery Investigation (GABI). N Engl J Med. 1994. 331:1037–1043.4. Kent KM, Bentivoglio LG, Block PC, et al. Longterm efficacy of percutaneous transluminal coronary angioplasty (PTCA): report from the National Heart, Lung, and Blood Institute PTCA Registry. Am J Cardiol. 1984. 53:27C–31C.5. Forrester JS, Fishbein M, Helfant R, Fagin J. A paradigm for restenosis based on cell biology: clues for the development of new preventive therapies. J Am Coll Cardiol. 1991. 17:758–769.6. Austin GE, Ratliff NB, Hollman J, Tabei S, Phillips DF. Intimal proliferation of smooth muscle cells as an explanation for recurrent coronary artery stenosis after percutaneous transluminal coronary angioplasty. J Am Coll Cardiol. 1985. 6:369–375.7. Bae Y, Jeong MH, Ahn YK, et al. Comparison of porcine coronary stent restenosis between MAC (Maximal Arterial Re-Creation) stent and Palmaz-Schatz stent. Korean Circ J. 1998. 28:89–96.8. Giraldo AA, Esposo OM, Meis JM. Intimal hyperplasia as a cause of restenosis after percutaneous transluminal coronary angioplasty. Arch Pathol Lab Med. 1985. 109:173–175.9. Ahn YK, Jeong MH, Kim JW, et al. Preventive effects of the heparin-coated stent on restenosis in the porcine model. Catheter Cardiovasc Interv. 1999. 48:324–330.10. Hong YJ, Jeong MH, Lim SY, et al. Elevated preprocedural high-sensitivity C-reactive protein levels are associated with neointimal hyperplasia and restenosis development after successful coronary artery stenting. Circ J. 2005. 69:1477–1483.11. The CAPTURE Investigators. Randomised placebo-controlled trial of abciximab before and during coronary intervention in refractory angina. Lancet. 1997. 349:1429–1435.12. The EPILOG Investigators. Platelet glycoprotein IIb / IIIa blockade and low-dose heparin during percutaneous coronary revascularisation. N Engl J Med. 1997. 336:1689–1696.13. Lefkovits J, Ivanhoe RJ, Califf RM, et al. Effects of platelet glycoprotein IIb/IIIa receptor blockade by a chimeric monoclonal antibody (abciximab) on acute and six-month outcomes after percutaneous transluminal coronary angioplasty for acute myocardial infarction. Am J Cardiol. 1996. 77:1045–1051.14. Topol EJ, Ferguson JJ, Weisman HF, et al. Long term protection from myocardial ischaemic events in a randomised trial of brief integrin beta3 blockade with percutaneous coronary intervention. JAMA. 1997. 278:479–484.15. Packer L, Witt EH, Tritschler HJ. Alpha-lipoic acid as a biological antioxidant. Free Radic Biol Med. 1995. 19:227–250.16. Packer L, Roy S, Sen CK. Alpha-lipoic acid: a metabolic antioxidant and potential redox modulator of transcription. Adv Pharmacol. 1997. 38:79–101.17. Song SJ, Kim KS, Park YJ, Jeong MH, Ko YM, Cho DL. Preparation of a dual-drug-eluting stent by grafting of ALA with abciximab on a bare metal stent. J Mater Chem. 2009. 19:8135–8141.18. Schwartz RS, Huber KC, Murphy JG, et al. Restenosis and the proportional neointimal response to coronary artery injury: results in a porcine model. J Am Coll Cardiol. 1992. 19:267–274.19. Schwartz RS, Edelman E, Virmani R, et al. Drug-eluting stents in preclinical studies: updated consensus recommendations for preclinical evaluation. Circ Cardiovasc Interv. 2008. 1:143–153.20. Kolodgie FD, John M, Khurana C, et al. Sustained reduction of in-stent neointimal growth with the use of a novel systemic nanoparticle paclitaxel. Circulation. 2002. 106:1195–1198.21. Lincoff AM. Potent complementary clinical benefit of abciximab and stenting during percutaneous coronary revascularization in patients with diabetes mellitus: results of the EPISTENT trial. Am Heart J. 2000. 139:S46–S52.22. Kang KT, Jeong MH, Kim NH, et al. The inhibitory effect of platelet glycoprotein IIb/IIIa receptor blocker-coated stent on porcine coronary stent restenosis. Korean J Med. 2001. 60:314–323.23. Park OY, Jeong MH, Kim JH, et al. The inhibitory effects of platelet glycoprotein IIb/IIIa receptor blocker-coated stent on neointima formation and inflammatory response in porcine coronary stent restenosis. Korean Circ J. 2003. 33:439–445.24. Hong YJ, Jeong MH, Kim W, et al. The effects of abciximab (Reopro(r))-coated stents on extracellular matrix synthesis and apoptosis. Korean Circ J. 2005. 35:290–301.25. Hong YJ, Jeong MH, Kim W, et al. Effect of abciximab-coated stent on in-stent intimal hyperplasia in human coronary arteries. Am J Cardiol. 2004. 94:1050–1054.26. Kim W, Jeong MH, Kim KH, et al. The clinical results of a platelet glycoprotein IIb/IIIa receptor blocker (abciximab: Reo-Pro)-coated stent in acute myocardial infarction. J Am Coll Cardiol. 2006. 47:933–938.27. Ziegler D, Hanefeld M, Ruhnau KJ, et al. Treatment of symptomatic diabetic polyneuropathy with the antioxidant alpha-lipoic acid: a 7-month multicenter randomized controlled trial (ALADIN III Study). ALADIN III Study Group. Alpha-Lipoic Acid in Diabetic Neuropathy. Diabetes Care. 1999. 22:1296–1301.28. Sung MJ, Kim W, Ahn SY, et al. Protective effect of alpha-lipoic acid in lipopolysaccharide-induced endothelial fractalkine expression. Circ Res. 2005. 97:880–890.29. Lim SY, Bae EH, Jeong MH, et al. The effect of alpha lipoic acid in a porcine in-stent restenosis model. J Cardiol. 2009. 54:375–385.30. Park OY, Jeong MH, Kim JH, et al. The role of extracellular matrix within the neointima in a porcine coronary stent restenosis model. Korean Circ J. 2003. 33:121–129.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Oral Administration of Alpha Lipoic Acid and Alpha Lipoic Acid Coated Stent in Porcine In-Stent Restenosis Model
- Anti-inflammatory Effect of Abciximab-Coated Stent in a Porcine Coronary Restenosis Model
- The preventive effects of the heparin-coated coronary stent in a porcine coronary stent restenosis model
- The preventive effects of the heparin-coated coronary stent in a porcine coronary stent restenosis model
- The Effects of the Heparin-Coated Maximum Arterial Re-Creation (MAC) Stent on Porcine Coronary Stent Restenosis