Allergy Asthma Immunol Res.
2016 Sep;8(5):421-427. 10.4168/aair.2016.8.5.421.
Systemic Reactions to Dust Mite Subcutaneous Immunotherapy: A 3-Year Follow-up Study
- Affiliations
-
- 1Department of Allergy, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China. zrf13092@163.com
- KMID: 2295151
- DOI: http://doi.org/10.4168/aair.2016.8.5.421
Abstract
- PURPOSE
The incidence of allergen specific immunotherapy-related systemic reactions (SRs) varies among different studies, and many factors are likely to contribute to SRs. This study aims to investigate the incidence, characteristics, and risk factors of SRs to standardize dust mite-specific subcutaneous immunotherapy (SCIT) in Central China.
METHODS
All patients receiving standardized dust mites (100-100,000 SQ-U/mL; Alutard SQ, Hørsholn, Denmark) immunotherapy were followed up. Recorded data included demographics, diagnosis, patient status, pulmonary function testing results before and after each injection, allergen dosage, and details of SRs.
RESULTS
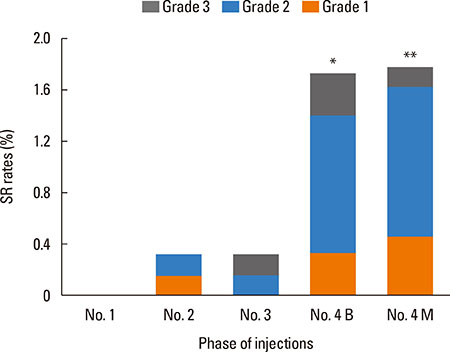
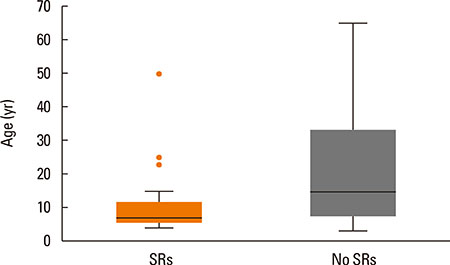
From June 2011 to August 2014, a total of 208 patients received 4,369 injections; 27 (13.0%) patients experienced 48 (1.1%) systemic reactions. Most of the SRs were grade 2 reactions (n=30, 62.5%), followed by grade 1 (n=11, 22.9%), grade 3 (n=7, 14.6%), and no fatal reactions occurred. Forty-six SRs (95.8%) occurred within 30 minutes. Higher SR rates were associated with high concentration extracts (100,000 SQ-U/mL), injections with concomitant local reactions (LRs), children, asthma and high sensitivity (skin prick test 3+/4+ and/or sIgE≥17.5 kUA/L) (P<0.05). The estimated odds of SRs increased in children (OR=6.57; 95% CI: 1.88-22.97, P=0.003), asthmatic patients (OR=4.10; 95% CI: 1.72-9.80, P=0.002), and injections with LRs (OR=2.41; 95% CI: 1.33-4.36, P=0.004).
CONCLUSIONS
The incidence of SRs to dust mite SCIT was low, and multiple factors were associated with the increased incidence of SRs. Children, asthmatics and patients with concomitant LR may be prone to develop SRs.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Efficacy of Sublingual Immunotherapy for House Dust Mite-Induced Allergic Rhinitis: A Meta-Analysis of Randomized Controlled Trials
Bohai Feng, Haijie Xiang, Haiyong Jin, Jinjian Gao, Saiyu Huang, Yunbin Shi, Ruru Chen, Bobei Chen
Allergy Asthma Immunol Res. 2017;9(3):220-228. doi: 10.4168/aair.2017.9.3.220.
Reference
-
1. Cox L, Nelson H, Lockey R, Calabria C, Chacko T, Finegold I, et al. Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol. 2011; 127:Suppl. S1–S55.2. Bousquet J, Lockey R, Malling HJ. Allergen immunotherapy: therapeutic vaccines for allergic diseases. A WHO position paper. J Allergy Clin Immunol. 1998; 102:558–562.3. Rank MA, Bernstein DI. Improving the safety of immunotherapy. J Allergy Clin Immunol Pract. 2014; 2:131–135.4. Li J, Huang Y, Lin X, Zhao D, Tan G, Wu J, et al. Factors associated with allergen sensitizations in patients with asthma and/or rhinitis in China. Am J Rhinol Allergy. 2012; 26:85–91.5. Roy SR, Sigmon JR, Olivier J, Moffitt JE, Brown DA, Marshall GD. Increased frequency of large local reactions among systemic reactors during subcutaneous allergen immunotherapy. Ann Allergy Asthma Immunol. 2007; 99:82–86.6. Cox L, Larenas-Linnemann D, Lockey RF, Passalacqua G. Speaking the same language: The World Allergy Organization Subcutaneous Immunotherapy Systemic Reaction Grading System. J Allergy Clin Immunol. 2010; 125:569–574.7. Alvarez-Cuesta E, Bousquet J, Canonica GW, Durham SR, Malling HJ, Valovirta E. Standards for practical allergen-specific immunotherapy. Allergy. 2006; 61:Suppl 82. 1–20.8. Burks AW, Calderon MA, Casale T, Cox L, Demoly P, Jutel M, et al. Update on allergy immunotherapy: American Academy of Allergy, Asthma & Immunology/European Academy of Allergy and Clinical Immunology/PRACTALL consensus report. J Allergy Clin Immunol. 2013; 131:1288–1296.e3.9. Zhou H, Tao QL, Wei JM, Xu G, Cheng L. Trends in specific immunotherapy for allergic rhinitis: a survey of Chinese ENT specialists. Allergy Asthma Immunol Res. 2014; 6:296–303.10. Rhee CS. Current specific immunotherapy for allergic rhinitis: perspectives from otorhinolaryngologists. Allergy Asthma Immunol Res. 2014; 6:273–275.11. Chen J, Li B, Zhao Y, Zhang Q, Wan L, Liu J, et al. A prospective multicenter study of systemic reactions in standardized specific immunotherapy for allergic rhinitis in China. Am J Rhinol Allergy. 2014; 28:e40–e44.12. Wang H, Lin X, Hao C, Zhang C, Sun B, Zheng J, et al. A double-blind, placebo-controlled study of house dust mite immunotherapy in Chinese asthmatic patients. Allergy. 2006; 61:191–197.13. DaVeiga SP, Caruso K, Golubski S, Lang DM. A Retrospective Survey of Systemic Reaction from Allergen Immunotherapy. J Allergy Clin Immunol. 2008; 121:suppl. S124.14. Lin MS, Tanner E, Lynn J, Friday GA Jr. Nonfatal systemic allergic reactions induced by skin testing and immunotherapy. Ann Allergy. 1993; 71:557–562.15. Rank MA, Oslie CL, Krogman JL, Park MA, Li JT. Allergen immunotherapy safety: characterizing systemic reactions and identifying risk factors. Allergy Asthma Proc. 2008; 29:400–405.16. Schiappoli M, Ridolo E, Senna G, Alesina R, Antonicelli L, Asero R, et al. A prospective Italian survey on the safety of subcutaneous immunotherapy for respiratory allergy. Clin Exp Allergy. 2009; 39:1569–1574.17. Winther L, Arnved J, Malling HJ, Nolte H, Mosbech H. Side-effects of allergen-specific immunotherapy: a prospective multi-centre study. Clin Exp Allergy. 2006; 36:254–260.18. Bernstein DI, Epstein T, Murphy-Berendts K, Liss GM. Surveillance of systemic reactions to subcutaneous immunotherapy injections: year 1 outcomes of the ACAAI and AAAAI collaborative study. Ann Allergy Asthma Immunol. 2010; 104:530–535.19. Epstein TG, Liss GM, Murphy-Berendts K, Bernstein DI. AAAAI/ACAAI surveillance study of subcutaneous immunotherapy, years 2008-2012: an update on fatal and nonfatal systemic allergic reactions. J Allergy Clin Immunol Pract. 2014; 2:161–167.20. Bernstein DI, Wanner M, Borish L, Liss GM. Immunotherapy Committee. American Academy of Allergy. Asthma and Immunology. Twelve-year survey of fatal reactions to allergen injections and skin testing: 1990-2001. J Allergy Clin Immunol. 2004; 113:1129–1136.21. Moreno C, Cuesta-Herranz J, Fernández-Távora L, Alvarez-Cuesta E. Immunotherapy Committee. Sociedad Española de Alergología e Inmunología Clínica. Immunotherapy safety: a prospective multi-centric monitoring study of biologically standardized therapeutic vaccines for allergic diseases. Clin Exp Allergy. 2004; 34:527–531.22. Kelso JM. The rate of systemic reactions to immunotherapy injections is the same whether or not the dose is reduced after a local reaction. Ann Allergy Asthma Immunol. 2004; 92:225–227.23. Tankersley MS, Butler KK, Butler WK, Goetz DW. Local reactions during allergen immunotherapy do not require dose adjustment. J Allergy Clin Immunol. 2000; 106:840–843.24. Calabria CW, Stolfi A, Tankersley MS. The REPEAT study: recognizing and evaluating periodic local reactions in allergen immunotherapy and associated systemic reactions. Ann Allergy Asthma Immunol. 2011; 106:49–53.25. Tabar AI, García BE, Rodríguez A, Olaguibel JM, Muro MD, Quirce S. A prospective safety-monitoring study of immunotherapy with biologically standardized extracts. Allergy. 1993; 48:450–453.26. Gamboa P, González G, Jauregui I, Jorró G, Molero I, Eseverri JL, et al. A prospective and multicenter safety-monitoring study of a short up-dosing schedule of immunotherapy with a mass-units-standardized extract of mites. Allergol Immunopathol (Madr). 2004; 32:13–17.27. DaVeiga SP, Liu X, Caruso K, Golubski S, Xu M, Lang DM. Systemic reactions associated with subcutaneous allergen immunotherapy: timing and risk assessment. Ann Allergy Asthma Immunol. 2011; 106:533–537.e2.28. Reid MJ, Lockey RF, Turkeltaub PC, Platts-Mills TA. Survey of fatalities from skin testing and immunotherapy 1985-1989. J Allergy Clin Immunol. 1993; 92:6–15.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect on quality of life of the mixed house dust mite/weed pollen extract immunotherapy
- The Effect of Specific Immunotherapy with House Dust Mite AIleI-gen in ChiIdhood Asthma
- Safety of Accelerated Schedules of Subcutaneous Allergen Immunotherapy with House Dust Mite Extract in Patients with Atopic Dermatitis
- Specific Antibody Response in House Dust Mite Asthmatics on Immunotherapy
- Patch test and Specific IgE Concentration with House Dust Mite Antigens in Atopic Dermatitis Patients