J Korean Acad Conserv Dent.
2010 May;35(3):211-221. 10.5395/JKACD.2010.35.3.211.
The effect of the removal of chondroitin sulfate on bond strength of dentin adhesives and collagen architecture
- Affiliations
-
- 1Department of Conservative Dentistry, School of Dentistry, Kyung Hee University, Seoul, Korea. choikkyu@khu.ac.kr
- KMID: 2294347
- DOI: http://doi.org/10.5395/JKACD.2010.35.3.211
Abstract
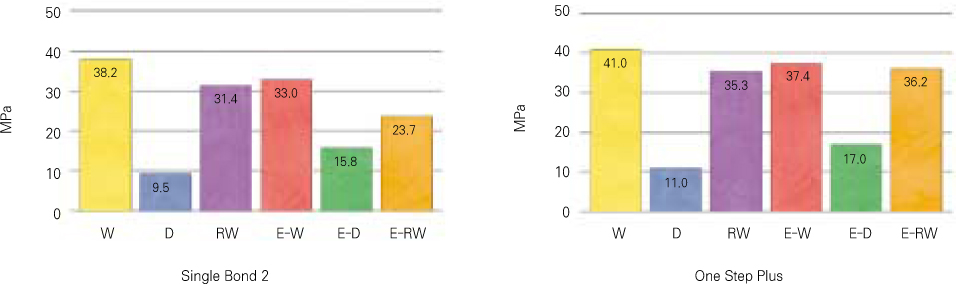
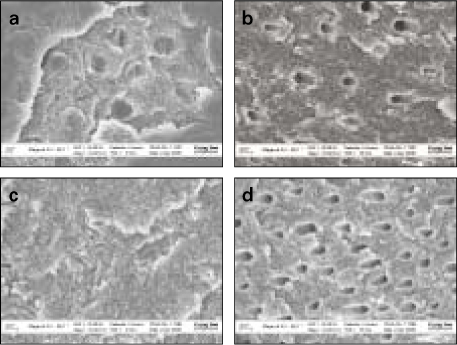
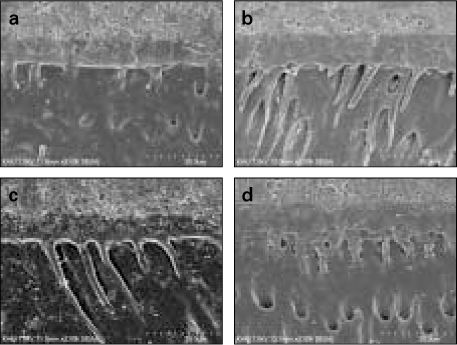
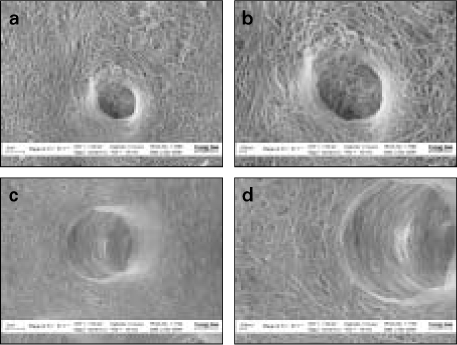
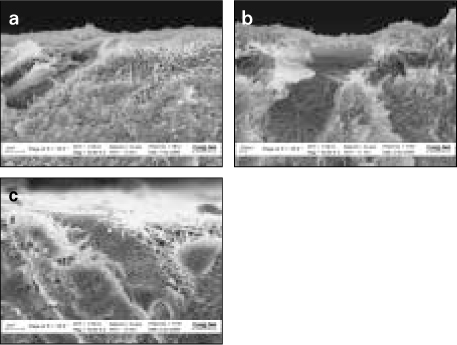
- Proteoglycan is highly hydrophilic and negatively charged which enable them attract the water. The objective of study was to investigate the effects of Proteoglycan on microtensile bond strength of dentin adhesives and on architecture of dentin collagen matrix of acid etched dentin by removing the chondroitin sulphate attached on Proteoglycan. A flat dentin surface in mid-coronal portion of tooth was prepared. After acid etching, half of the specimens were immersed in 0.1 U/mL chondroitinase ABC (C-ABC) for 48 h at 37degrees C, while the other half were stored in distilled water. Specimens were bonded with the dentin adhesive using three different bonding techniques (wet, dry and re-wet) followed by microtensile bond strength test. SEM examination was done with debonded specimen, resin-dentin interface and acid-etched dentin surface with/without C-ABC treatment. For the subgroups using wet-bonding or dry-bonding technique, microtensile bond strength showed no significant difference after C-ABC treatment (p > 0.05). Nevertheless, the subgroup using rewetting technique after air dry in the Single Bond 2 group demonstrated a significant decrease of microtensile bond strength after C-ABC treatment. Collagen architecture is loosely packed and some fibrils are aggregated together and relatively collapsed compared with normal acid-etched wet dentin after C-ABC treatment. Further studies are necessary for the contribution to the collagen architecture of noncollagenous protein under the various clinical situations and several dentin conditioners and are also needed about long-term effect on bond strength of dentin adhesive.
Keyword
MeSH Terms
Figure
Reference
-
1. Van Meerbeek B, De Munck J, Yoshida Y, Inoue S, Vargas M, Vijay P, Van Landuyt K, Lambrechts P, Vanherle G. Buonocore memorial lecture. Adhesion to enamel and dentin: current status and future challenges. Oper Dent. 2003. 28(3):215–235.2. Nakabayashi N, Kojima K, Masuhara E. The promotion of adhesion by the infiltration of monomers into tooth substrates. J Biomed Mater Res. 1982. 16(3):265–273.
Article3. Wang Y, Spencer P. Quantifying adhesive penetration in adhesive/dentin interface using confocal Raman microspectroscopy. J Biomed Mater Res. 2002. 59(1):46–55.
Article4. Sano H, Takatsu T, Ciucchi B, Horner JA, Matthews WG, Pashley DH. Nanoleakage: leakage within the hybrid layer. Oper Dent. 1995. 20(1):18–25.5. Jang JH, Lee KW, Kim HY, Lee IB, Cho BH, Son HH. Quatitative comparision of permeability in the adhesive interface of four adhesive systems. J Kor Acad Con Dent. 2009. 34(1):55–60.6. Son SJ, Jang JH, Kang SH, Yoo HM, Cho BH, Son HY. The nanoleakage patterns of experimental hydrophobic adhesives after load cycling. J Kor Acad Cons Dent. 2008. 33(1):9–19.
Article7. Perdigao J, Van Meerbeek B, Lopes MM, Ambrose WW. The effect of a re-wetting agent on dentin bonding. Dent Mater. 1999. 15(4):282–295.
Article8. Gwinnett AJ. Dentin bond strength after air drying and rewetting. Am J Dent. 1994. 7(3):144–148.9. Carvalho RM, Yoshiyama M, Pashley EL, Pashley DH. In vitro study on the dimensional changes of human dentine after demineralization. Arch Oral Biol. 1996. 41(4):369–377.
Article10. Pashley DH, Agee KA, Nakajima M, Tay FR, Carvalho RM, Terada RS, Harmon FJ, Lee WK, Rueggeberg FA. Solvent-induced dimensional changes in EDTA-demineralized dentin matrix. J Biomed Mater Res. 2001. 56(2):273–281.
Article11. Agee KA, Becker TD, Joyce AP, Rueggeberg FA, Borke JL, Waller JL, Tay FR, Pashley DH. Net expansion of dried demineralized dentin matrix produced by monomer/alcohol saturation and solvent evaporation. J Biomed Mater Res A. 2006. 79(2):349–358.
Article12. Linde A. Dentin matrix proteins: composition and possible functions in calcification. Anat Rec. 1989. 224(2):154–166.
Article13. Chaussain-Miller C, Fioretti F, Goldberg M, Menashi S. The role of matrix metalloproteinases (MMPs) in human caries. J Dent Res. 2006. 85(1):22–32.
Article14. Orsini G, Ruggeri A Jr, Mazzoni A, Papa V, Mazzotti G, Di Lenarda R, Breschi L. Immunohistochemical identification of decorin and biglycan in human dentin: a correlative field emission scanning electron microscopy/transmission electron microscopy study. Calcif Tissue Int. 2007. 81(1):39–45.
Article15. Goldberg M, Takagi M. Dentine proteoglycans: composition, ultrastructure and functions. Histochem J. 1993. 25(11):781–806.
Article16. Scott JE. Proteoglycan:collagen interactions and subfibrillar structure in collagen fibrils. Implications in the development and ageing of connective tissues. J Anat. 1990. 169(1):23–35.17. Ho SP, Sulyanto RM, Marshall SJ, Marshall GW. The cementum-dentin junction also contains glycosaminoglycans and collagen fibrils. J Struct Biol. 2005. 151(1):69–78.
Article18. Hall R, Septier D, Embery G, Goldberg M. Stromelysin-1 (MMP-3) in forming enamel and predentine in rat incisor-coordinated distribution with proteoglycans suggests a functional role. Histochem J. 1999. 31(12):761–770.19. Oh EW, Choi KK, Kim JR, Park SJ. Effect of chlohexidine on microtensile bond strength of dentin bonding systems. J Kor Acad Cons Dent. 2008. 33(2):148–161.
Article20. Smith AJ, Wade W, Addy M, Embery G. The relationship between microbial factors and gingival crevicular fluid glycosaminoglycans in human adult periodontitis. Arch Oral Biol. 1997. 42(1):89–92.
Article21. Breschi L, Lopes M, Gobbi P, Mazzotti G, Falconi M, Perdigao J. Dentin proteoglycans: an immunocytochemical FEISEM study. J Biomed Mater Res. 2002. 61(1):40–46.
Article22. Van Meerbeek B, Vargas M, Inoue S, Yoshida Y, Perdigao J, Lambrechts P, Vanherle G. Microscopy investigations. Techniques, results, limitations. Am J Dent. 2000. 13(Spec No):3D–18D.23. Perdigao J, Lambrechts P, Van Meerbeek B, Vanherle G, Lopes AL. Field emission SEM comparison of four postfixation drying techniques for human dentin. J Biomed Mater Res. 1995. 29(9):1111–1120.
Article24. Embery G, Hall R, Waddington R, Septier D, Goldberg M. Proteoglycans in dentinogenesis. Crit Rev Oral Biol Med. 2001. 12(4):331–349.
Article25. Goldberg M, Septier DS. Differential staining of glycosaminoglycans in the predentine and dentine of rat incisor using cuprolinic blue at various magnesium chloride concentrations. Histochem J. 1992. 24(9):648–654.
Article26. Hall RC, Embery G, Lloyd D. Immunochemical localization of the small leucine-rich proteoglycan lumican in human predentine and dentine. Arch Oral Biol. 1997. 42(10-11):783–786.
Article27. Kagayama M, Sasano Y, Mizoguchi I, Kamo N, Takahashi I, Mitani H. Localization of glycosaminoglycans in periodontal ligament during physiological and experimental tooth movement. J Periodontal Res. 1996. 31(4):229–234.
Article28. Yamamoto T, Domon T, Takahashi S, Islam MN, Suzuki R. The fibrous structure of the cemento-dentinal junction in human molars shown by scanning electron microscopy combined with NaOH-maceration. J Periodontal Res. 2000. 35(2):59–64.
Article29. Scott JE. Proteoglycan-fibrillar collagen interactions. Biochem J. 1988. 252:313–323.
Article30. Raspanti M, Congiu T, Alessandrini A, Gobbi P, Ruggeri A. Different patterns of collagen-proteoglycan interaction: a scanning electron microscopy and atomic force microscopy study. Eur J Histochem. 2000. 44(4):335–343.31. Bartold PM, Miki Y, McAllister B, Narayanan AS, Page RC. Glycosaminoglycans of human cementum. J Periodontal Res. 1988. 23(1):13–17.
Article32. Redaelli A, Vesentini S, Soncini M, Vena P, Mantero S, Montevecchi FM. Possible role of decorin glycosaminoglycans in fibril to fibril force transfer in relative mature tendons--a computational study from molecular to microstructural level. J Biomech. 2003. 36(10):1555–1569.
Article33. Mazzoni A, Pashley DH, Ruggeri A Jr, Vita F, Falconi M, Di Lenarda R, Breschi L. Adhesion to chondroitinase ABC treated dentin. J Biomed Mater Res B Appl Biomater. 2008. 86(1):228–236.
Article34. Pereira PN, Bedran-de-Castro AK, Duarte WR, Yamauchi M. Removal of noncollagenous components affects dentin bonding. J Biomed Mater Res B Appl Biomater. 2007. 80(1):86–91.
Article35. Elliott DM, Robinson PS, Gimbel JA, Sarver JJ, Abboud JA, Iozzo RV, Soslowsky LJ. Effect of altered matrix proteins on quasilinear viscoelastic properties in transgenic mouse tail tendons. Ann Biomed Eng. 2003. 31(5):599–605.
Article36. Perdigao J, Frankenberger R. Effect of solvent and rewetting time on dentin adhesion. Quintessence Int. 2001. 32(5):385–390.37. Pashley DH, Tay FR, Carvalho RM, Rueggeberg FA, Agee KA, Carrilho M, Donnelly A, Garcia-Godoy F. From dry bonding to water-wet bonding to ethanol-wet bonding. A review of the interactions between dentin matrix and solvated resins using a macromodel of the hybrid layer. Am J Dent. 2007. 20(1):7–20.38. Van Landuyt KL, Snauwaert J, De Munck J, Peumans M, Yoshida Y, Poitevin A, Coutinho E, Suzuki K, Lambrechts P, Van Meerbeek B. Systematic review of the chemical composition of contemporary dental adhesives. Biomaterials. 2007. 28(26):3757–3785.
Article39. Peters WJ, Jackson RW, Smith DC. Studies of the stability and toxicity of zinc polyacrylate (polycarboxylate) cements (PAZ). J Biomed Mater Res. 1974. 8(1):53–60.
Article40. Van Meerbeek B, Conn LJ Jr, Duke ES, Eick JD, Robinson SJ, Guerrero D. Correlative transmission electron microscopy examination of nondemineralized and demineralized resin-dentin interfaces formed by two dentin adhesive systems. J Dent Res. 1996. 75(3):879–888.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The effect of bonding resin on bond strength of dual-cure resin cements
- Bonding of a resin-modified glass ionomer cement to dentin using universal adhesives
- The effect of cyanate methacrylate on the shear bond strengths to dentin
- Effect of additional etching and ethanol-wet bonding on the dentin bond strength of one-step self-etch adhesives
- Microtensile bond strength of single step adhesives to dentin