Korean J Urol.
2006 Nov;47(11):1155-1160. 10.4111/kju.2006.47.11.1155.
Anticancer Activity of Intravesical Glyceryl Monooleate (GMO)-Paclitaxel Therapy in Murine Superficial Transitional Cell Carcinoma Model Induced by BBN
- Affiliations
-
- 1Department of Urology, The Catholic University of Korea, Seoul, Korea.
- KMID: 2294272
- DOI: http://doi.org/10.4111/kju.2006.47.11.1155
Abstract
- Purpose
Paclitaxel, on systemic administration, is widely known to be effective in the treatment of bladder cancer. However, the intravesical use of paclitaxel has not been attempted because of its liposolubility and direct toxicity to the bladder mucosa. The purpose of this study was to evaluate the efficacy and toxicity of paclitaxel-loaded glyceryl monooleate (GMO) in the intravesical treatment of superficial bladder cancer, by enhancing its bioadhesiveness and bioavailability.
Materials and Methods
12 mice were divided into two groups, and bladder carcinomas induced by the addition of 0.05% BBN to their drinking water for 12 weeks. Group 1 received an intravesical instillation of 0.1ml GMO-paclitaxel-free buffer and Group 2 an intravesical instillation of 0.1ml GMO-paclitaxel. On day 21, the tumor incidence, bladder weight and toxicity were evaluated.
Results
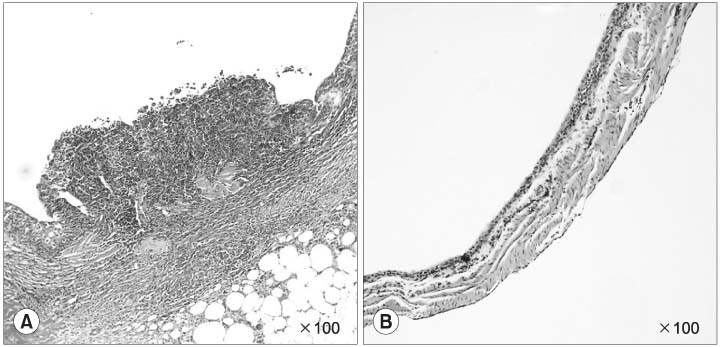
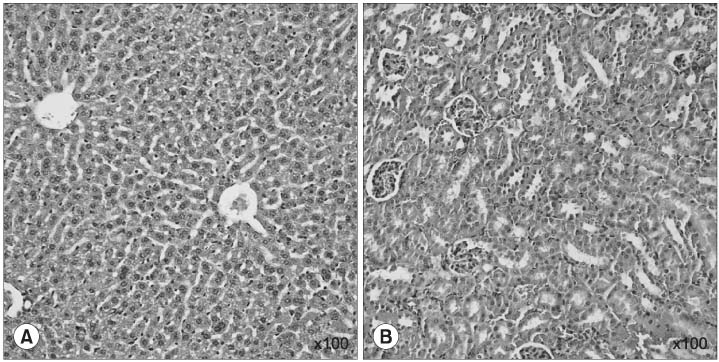
The incidence of cancer in groups 1 and 2 were 83 and 17%, respectively. The incidence of cancer was significantly reduced in group 2 compared to group 1 (p<0.05). There was a tendency for the average bladder weight in group 1 to be heavier than that in group 2, but there was no significant difference (p=0.375). There were no liver, kidney or bone marrow toxicities in either group.
Conclusions
Intravesical GMO-paclitaxel therapy may have an inhibitory effect on the growth of superficial bladder cancer in a BBN-induced bladder cancer model; therefore, it could potentially be used in those patients showing little to no response to intravesical Bacillus Calmette- Guerin (BCG) or other anticancer drug therapies.
MeSH Terms
Figure
Reference
-
1. Lamm DL. Long-term results of intravesical therapy for superficial bladder cancer. Urol Clin North Am. 1992. 19:573–580.2. Traynelis CL, Lamm DL. Rous SN, editor. Current status of intravesical therapy for bladder cancer. Urology annual. 1994. East Norwalk: Appleton & Lange;113–143.3. Badalament RA, Farah RN. Treatment of superficial bladder cancer with intravesical chemotherapy. Semin Surg Oncol. 1997. 13:335–341.4. Nadler RB, Catalona WJ, Hudson MA, Ratliff TL. Durability of the tumor free response for intravesical bacillus Calmette-Guerin therapy. J Urol. 1994. 152:367–373.5. Nseyo UO, Lamm DL. Immunotherapy of bladder cancer. Semin Surg Oncol. 1997. 13:342–349.6. Herr HW. Tumour progression and survival in patients with T1G3 bladder tumours: 15-year outcome. Br J Urol. 1997. 80:762–765.7. Engstrom S, Norden TP, Nyquist H. Cubic phases for studies of drug partition into lipid bilayers. Eur J Pharm Sci. 1999. 8:243–254.8. Lee SJ, Chung CE, Kim SE, Lee CB, Kang SH, Cho YH, et al. Efficacy of paclitaxel-loaded bioadhesive drug deliver system based on glyceryl monooleate nanoparticle in an orthotopic murine bladder cancer model. Korean J Urol. 2004. 45:817–822.9. Helledi LS, Schubert L. Release kinetics of acyclovir from a suspension of acyclovir incorporated in a cubic phase delivery system. Drug Dev Ind Pharm. 2001. 27:1073–1081.10. Longer M, Tyle P, Mauger J. A cubic-phase oral drug delivery system for controlled release of AG337. Drug Dev Ind Pharm. 1996. 22:603–608.11. Lee CH, Sohn DW, Kim HS, Lee SJ, Cho YH, Yoon MS, et al. The anticancer efficacy and toxicity of oral paclitaxel-loaded lipid nanoparticle in a C3H2 bladder cancer mice. Korean J Urol. 2005. 46:854–860.12. Kim DB, Jang J, Cho YH, Yoon MS, Chung HS, Park YT, et al. Anticancer efficacy and toxicity of oral GMO-paclitaxel in a hormone refractory prostate cancer model. Korean J Urol. 2006. 47:143–149.13. Lee C, Lee ES, Choi H, Koh SK, Lee JM, Chai SE, et al. Incidence estimation of genitourinary cancer in Korea. J Korean Med Sci. 1992. 7:154–161.14. Lee SC. Intravesical therapy for superficial bladder cancer: advances and future. Korean J Urol. 2000. 41:467–479.15. Steinberg GD, Brendler CB, Squire RA, Isaacs JT. Experimental intravesical therapy for superficial transitional cell carcinoma in a rat bladder tumor model. J Urol. 1991. 145:647–653.16. Fukushima S, Murasaki G, Hirose M, Nakanishi K, Hasegawa R, Ito N. Histopathological analysis of preneoplastic changes during N-butyl-N-(4-hydroxybutyl)nitrosamine-induced urinary bladder carcinogenesis in rats. Acta Pathol Jpn. 1982. 32:243–250.17. Bisson JF, Parache RM, Droulle P, Notter D, Vigneron C, Guillemin F. A new method of implanting orthotopic rat bladder tumor for experimental therapies. Int J Cancer. 2002. 102:280–285.18. Ito N, Matayoshi M, Arai M, Yoshioka Y, Kamamoto Y. Effect of various factors on induction of urinary bladder tumors in animals by N-butyl-N-(4-hydroxybutyl)nitrosamine. Gann. 1973. 64:151–159.19. Kim HG, Park IA, Yeon WG, Yoon SJ, Byun SS, Lee ES, et al. Apoptosis and expressions of apoptosis-related proteins in rat bladder carcinogenesis induced by N-butyl-N-(4-hydroxybutyl) nitrosamine. Korean Urol Oncol Soc. 2003. 1:106–111.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Efficacy of Paclitaxel-Loaded Bioadhesive Drug Deliver System Based on Glyceryl Monooleate Nanoparticle in an Orthotopic Murine Bladder Cancer Model
- Anticancer Efficacy and Toxicity of Oral GMO-paclitaxel in a Hormone Refractory Prostate Cancer Model
- Bioadhesive Drug Delivery System for the Intravesical Administration of Paclitaxel in Rabbits
- Intravesical Chemotherpy in Superficial Transitional Cell Carcinoma of the Bladder Preliminary Report
- Effect of Intraperitoneal and Intravesical Bacillus Calmette-Guerin on Bladder Carcinogenesis in Rats Induced by N-butyl-N-(4-hydroxybutyl) nitrosamine