Korean J Urol.
2006 May;47(5):467-474. 10.4111/kju.2006.47.5.467.
The Effects of GAC on the Biochemical Profiles and Quality of Life of Metastatic Prostate Cancer Patients
- Affiliations
-
- 1Department of Urology, Urological Science Institute and Brain Korea 21 Project for Medical Science, Seoul, Korea. sjhong346@yumc.yonsei.ac.kr
- 2Department of Food and Nutrition, Yonsei University, Seoul, Korea.
- 3Research Institute of Food and Nutritional Sciences, Yonsei University, Seoul, Korea.
- 4R&D Division, Amino Up Chemical Company, Sapporo, Japan.
- KMID: 2294129
- DOI: http://doi.org/10.4111/kju.2006.47.5.467
Abstract
- PURPOSE
In order to evaluate the effects of GAC, which is the combination of active hexose correlated compound (AHCC) and genistein combined polysaccharide (GCP), we investigated the changes in the biochemical profiles and the quality of life of prostate cancer patients with androgen suppression after the administration of GAC.
MATERIALS AND METHODS
Thirty two eligible metastatic prostate cancer patients between the ages of 54 and 84 were enrolled in this study, and they were supplemented with 5g GAC per day (n=23) or placebo (n=9) for a 6 months period. Blood and urine sample analysis were taken and the quality of life (QoL) was assessed using the Visual Analogue Scale (VAS) and the Functional Assessment of Cancer Therapy Scale Questionnaire (FACT-G) at baseline and at post intervention (after 3 and 6 months).
RESULTS
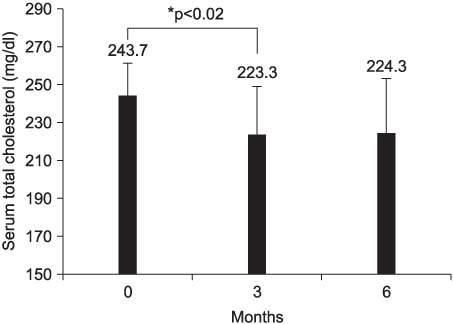
Twenty six patients (n=18 in the GAC group and n=8 in the placebo group) completed the 6 months intervention. No statistically significant adverse events were reported by the study participants. GAC had no significant effect on the serum biochemical parameters. However, all 7 GAC-treated hypercholesterolemic patients had their cholesterol level decreased after 3 months treatment (p<0.02). Results of Comet assay showed significant decreases in tail moment (p<0.009) and tail length (p<0.004) at 6 months compared to baseline for the GAC group. Although the results of the VAS were inconsistent, the score for physical well-being was increased in GAC group on the FACT-G analysis (p<0.05 between baseline and 3 months, respectively).
CONCLUSIONS
Oral administration of GAC 5g per day for 6 months showed a decrease in DNA damage of blood lymphocytes and in the total serum cholesterol level in hypercholesterolemic patients without any significant influences on the serum biochemical parameters of the metastatic prostate cancer patients. Further studies on the role of GAC are necessary to clarify the advantage of GAC supplementation in prostate cancer patients with androgen suppression.
MeSH Terms
Figure
Cited by 1 articles
-
Complementary Therapy for Improvement of Quality of Life in Cancer Patients
Jun Young Choi
J Korean Med Assoc. 2008;51(5):435-448. doi: 10.5124/jkma.2008.51.5.435.
Reference
-
1. Parker SL, Tong T, Bolden S, Wingo PA. Cancer statistics, 1997. CA Cancer J Clin. 1997. 47:5–27.2. Catalona WJ, Smith DS, Ratliff TL, Basler JW. Detection of organ-confined prostate cancer is increased through prostate specific antigen-based screening. JAMA. 1993. 270:948–954.3. Epstein JI. Incidence and significance of positive margins in radical prostatectomy specimens. Urol Clin North Am. 1996. 23:651–663.4. Stege R. Potential side-effects of endocrine treatment of long duration in prostate cancer. Prostate. 2000. 10:Suppl. 38–42.5. Hong SJ, Kim SI, Kwon SM, Lee JR, Chung BC. Comparative study of concentration of isoflavones and lignans in plasma and prostatic tissues of normal control and benign prostatic hyperplasia. Yonsei Med J. 2002. 43:235–241.6. Messina MJ, Persky V, Setchell KD, Barnes S. Soy intake and cancer risk:a review of the in vitro and in vivo data. Nutr Cancer. 1994. 21:113–130.7. Geller J, Sionit L, Partido C, Li L, Tan X, Youngkin T, et al. Genistein inhibits the human patient BPH and prostate cancer in histoculture. Prostate. 1998. 34:75–79.8. Bemis DL, Capodice JL, Desai M, Buttyan R, Katz AE. A concentrated aglycone isoflavone preparation (GCP) that demonstrates potent anti-prostate cancer activity in vitro and in vivo. Clin Cancer Res. 2004. 10:5282–5292.9. Matsushita K, Kuramitsu Y, Ohiro Y, Obara M, Kobayashi M, Li YO. Combination therapy of active hexose correlated compound plus UFT significantly reduces the metastasis of rat mammary adenocarcinoma. Anticancer Drugs. 1998. 9:343–350.10. Yagita A, Maruyama S, Wakasugi S, Sukegawa Y. H-2 haplotype-dependent serum IL-12 production in tumor-bearing mice treated with various mycelial extracts. In Vivo. 2002. 16:49–54.11. Matsui Y, Uhara J, Satoi S, Kaibori M, Yamada H, Kitade H, et al. Improved prognosis of postoperative hepatocellular carcinoma patients when treated with functional foods: a prosptective cohort study. J Hepatol. 2002. 37:78–86.12. Lin AD, Chen KK, Lin AT, Chang YH, Wu HH, Kuo JY, et al. Antiandrogen-associated hepatotoxicity in the management of advanced prostate cancer. J Chin Med Assoc. 2003. 66:735–740.13. Betti C, Davini T, Giannessi L, Loprieno N, Barale R. Micro gel electorphoresis assay (comet assay) and SCE analysis in human lymphocytes from 100 normal subjects. Mutat Res. 1994. 307:323–333.14. Xu X, Harris KS, Wang HJ, Murphy PA, Hendrich S. Bioavilability of soybean isoflavones depends upon gut microflora in women. J Nutr. 1995. 125:2307–2315.15. Rafii F, Davis C, Park M, Heinze TM, Beger RD. Variation in metabolism of the soy isoflavonoid daidzein by human intestinal microfloras from different individuals. Arch Microbiol. 2003. 180:11–16.16. Rowland IR, Wiseman H, Sanders TA, Adlercreutz H, Bowey EA. Interindividual variation in metabolism of soy isoflavones and lignans: influence of habital diet on equol production by the gut microflora. Nutr Cancer. 2000. 36:27–32.17. Urban D, Irwin W, Kirk M, Markiewicz MA, Myers R, Smith M, et al. The effect of isolated soy protein on plasma biomarkers in elderly men with elevated serum prostate specific antigen. J Urol. 2001. 165:294–300.18. Demark-Wahnefried W, Price DT, Polascik TJ, Robertson CN, Anderson EE, Walther PJ, et al. Pilot study of dietary fat restriction and flaxseed supplementation in men with prostate cancer before surgery: exploring the effects on hormonal levels, prostate-specific antigen, and histopathologic features. Urology. 2001. 58:47–52.19. Demark-Wahnefried W, Robertson CN, Walther PJ, Polascik TJ, Paulson DF, Vollmer RT. Pilot study to explore effects of low-fat, flaxseed-supplemented diet on proliferation of benign prostatic epithelium and prostate-specific antigen. Urology. 2004. 63:900–904.20. Hong SJ, Kim JS, Lee MJ, Yoon S, Lee JM, Oh HY. The effect of isoflavone intake on serum biochemical profiles and antioxidant system in patients with prostatic Diseases. Korean J Urol. 2005. 46:360–365.21. Freeman MR, Solomon KR. Cholesterol and prostate cancer. J Cell Biochem. 2004. 91:54–69.22. Zhuang L, Kim J, Adam RM, Solomon KR, Freeman MR. Cholesterol targeting alters lipid raft composition and cell survival in prostate cancer cells and xenografts. J Cin Invest. 2005. 115:959–968.23. Ye SF, Ichimura K, Wakame K, Ohe M. Suppressive effects of Active Hexose Correlated Compound on the increased activity of hepatic and renal ornithine decarboxylase induced by oxidative stress. Life Sci. 2003. 74:593–602.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Dexamethasone Treatment of Bone Pain in Metastatic Prostate Cancer: Assessment of Pain Indices and Quality of Life
- Quality of Life in Prostate Cancer Patient Undergoing Androgen Deprivation Therapy
- Factors Influencing the Quality of Life of Prostate Cancer Patients
- Improved Quality of Life (QOL) During the Off-treatment Intervals of the Intermittent Androgen Deprivation (IAD) in the Prostate Cancer Patients
- Relationship of Urinary Symptom, Urinary Discomfort and Quality of Life in Bladder Cancer and Benign Prostatic Hypertrophy of Male Patients