J Gynecol Oncol.
2013 Apr;24(2):177-185. 10.3802/jgo.2013.24.2.177.
The mechanism of mesna in protection from cisplatin-induced ovarian damage in female rats
- Affiliations
-
- 1Department of Histology and Embryology, School of Basic Medical Sciences, Capital Medical University, Beijing, China. zhoudeshan2008@163.com
- KMID: 2288550
- DOI: http://doi.org/10.3802/jgo.2013.24.2.177
Abstract
OBJECTIVE
Cisplatin is a widely used chemotherapeutic agent in the treatment of cancers in clinic; but it often induces adverse effects on ovarian functions such as reduced fertility and premature menopause. Mesna could attenuate the cisplatin-induced ovarian damages; however, the underlying mechanism is still unknown. This study aimed to figure out the underlying mechanism of the protection of mesna for ovaries against cisplatin therapy in cancers.
METHODS
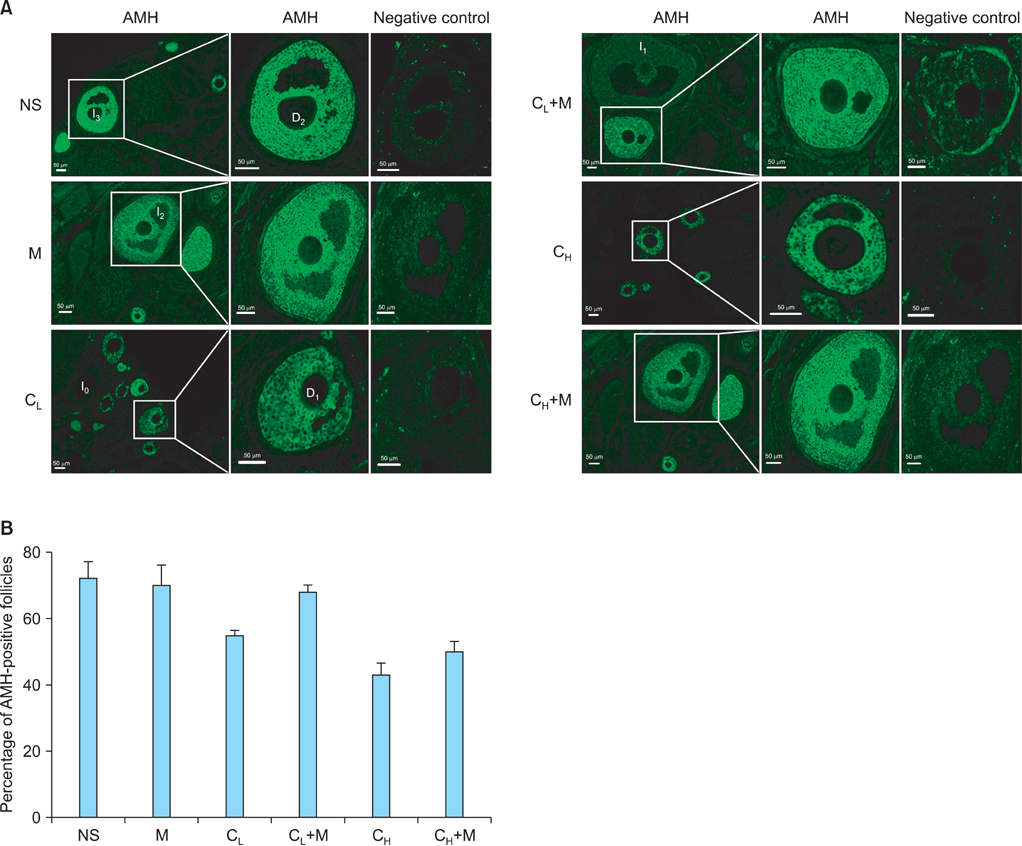
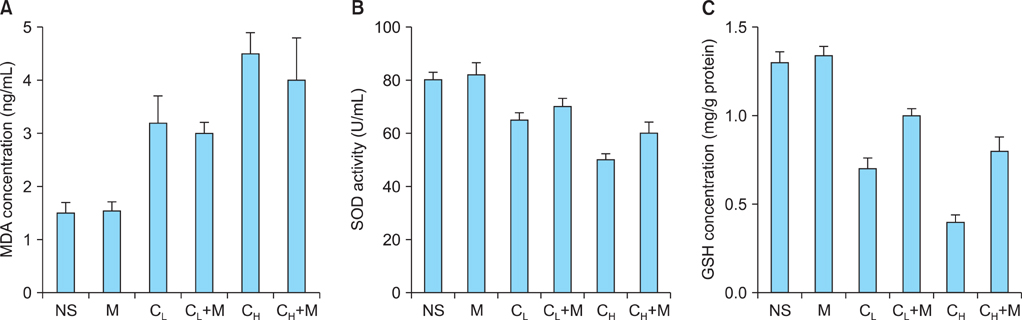
We performed female adult Sprague-Dawley rats into normal saline control (NS), low-dose cisplatin (CL), high-dose cisplatin (CH), CL plus mesna (CL+M), and CH plus mesna (CH+M) groups and detected anti-Mullerian hormone (AMH)-positive follicle, oxidative stress status and anti-oxidative capability in ovaries.
RESULTS
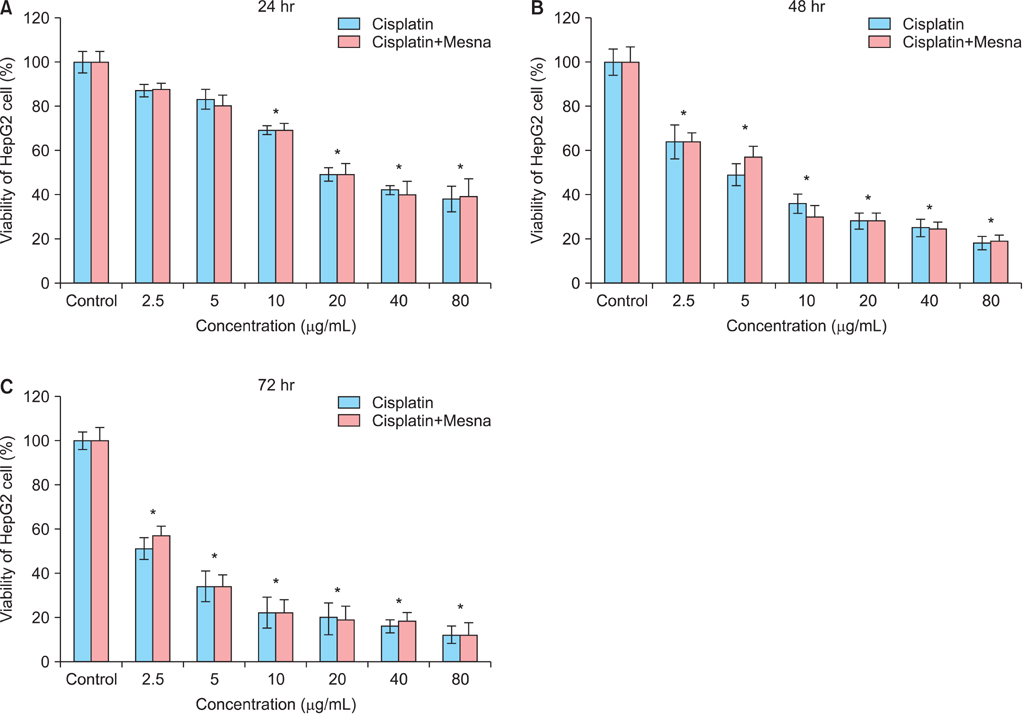
AMH-positive follicles were significantly decreased after cisplatin administration, which was significantly reversed when mesna was co-administered with cisplatin. The end product of lipid peroxidation, malondialdehyde (MDA), was significantly increased, but the anti-oxidative enzymatic activity of superoxide dismutase (SOD) and glutathione (GSH) were significantly decreased in cisplatin groups when compared with NS group. In contrast, after co-administration of cisplatin with mesna, MDA was significantly decreased whereas the activity of SOD and the concentration of GSH were increased. Moreover, mesna did not decrease the anti-tumor property of cisplatin in HePG2 cell lines.
CONCLUSION
Cisplatin damages the granulosa cells by oxidative stress to deplete the ovarian reserve and mesna could protect ovarian reserve through anti-oxidation. These results might highlight the mechanism of the protection of mesna for ovarian reserve and open an avenue for the application of mesna as a protective additive in cisplatin chemotherapy in clinical practise.
MeSH Terms
-
Adult
Animals
Anti-Mullerian Hormone
Cisplatin
Female
Fertility
Glutathione
Granulosa Cells
Hep G2 Cells
Humans
Lipid Peroxidation
Malondialdehyde
Menopause, Premature
Mesna
Ovary
Oxidative Stress
Rats
Rats, Sprague-Dawley
Superoxide Dismutase
Anti-Mullerian Hormone
Cisplatin
Glutathione
Malondialdehyde
Mesna
Superoxide Dismutase
Figure
Reference
-
1. Meirow D. Reproduction post-chemotherapy in young cancer patients. Mol Cell Endocrinol. 2000. 169:123–131.2. Lebwohl D, Canetta R. Clinical development of platinum complexes in cancer therapy: an historical perspective and an update. Eur J Cancer. 1998. 34:1522–1534.3. Berger T, Malayeri R, Doppelbauer A, Krajnik G, Huber H, Auff E, et al. Neurological monitoring of neurotoxicity induced by paclitaxel/cisplatin chemotherapy. Eur J Cancer. 1997. 33:1393–1399.4. Li Y, Womer RB, Silber JH. Predicting cisplatin ototoxicity in children: the influence of age and the cumulative dose. Eur J Cancer. 2004. 40:2445–2451.5. Townsend DM, Tew KD, He L, King JB, Hanigan MH. Role of glutathione S-transferase Pi in cisplatin-induced nephrotoxicity. Biomed Pharmacother. 2009. 63:79–85.6. Yucebilgin MS, Terek MC, Ozsaran A, Akercan F, Zekioglu O, Isik E, et al. Effect of chemotherapy on primordial follicular reserve of rat: an animal model of premature ovarian failure and infertility. Aust N Z J Obstet Gynaecol. 2004. 44:6–9.7. Lutchman Singh K, Davies M, Chatterjee R. Fertility in female cancer survivors: pathophysiology, preservation and the role of ovarian reserve testing. Hum Reprod Update. 2005. 11:69–89.8. Sklar CA, Mertens AC, Mitby P, Whitton J, Stovall M, Kasper C, et al. Premature menopause in survivors of childhood cancer: a report from the childhood cancer survivor study. J Natl Cancer Inst. 2006. 98:890–896.9. Maltaris T, Seufert R, Fischl F, Schaffrath M, Pollow K, Koelbl H, et al. The effect of cancer treatment on female fertility and strategies for preserving fertility. Eur J Obstet Gynecol Reprod Biol. 2007. 130:148–155.10. Wallace WH, Shalet SM, Crowne EC, Morris-Jones PH, Gattamaneni HR, Price DA. Gonadal dysfunction due to cis-platinum. Med Pediatr Oncol. 1989. 17:409–413.11. Stroud JS, Mutch D, Rader J, Powell M, Thaker PH, Grigsby PW. Effects of cancer treatment on ovarian function. Fertil Steril. 2009. 92:417–427.12. Nasir J, Walton C, Lindow SW, Masson EA. Spontaneous recovery of chemotherapy-induced primary ovarian failure: implications for management. Clin Endocrinol (Oxf). 1997. 46:217–219.13. Evans DL, Dive C. Effects of cisplatin on the induction of apoptosis in proliferating hepatoma cells and nonproliferating immature thymocytes. Cancer Res. 1993. 53:2133–2139.14. Cepeda V, Fuertes MA, Castilla J, Alonso C, Quevedo C, Perez JM. Biochemical mechanisms of cisplatin cytotoxicity. Anticancer Agents Med Chem. 2007. 7:3–18.15. Rybak LP, Whitworth CA, Mukherjea D, Ramkumar V. Mechanisms of cisplatin-induced ototoxicity and prevention. Hear Res. 2007. 226:157–167.16. Choi J, Im GJ, Chang J, Chae SW, Lee SH, Kwon SY, et al. Protective effects of apocynin on cisplatin-induced ototoxicity in an auditory cell line and in zebrafish. J Appl Toxicol. 2013. 33:125–133.17. Satoh M, Kashihara N, Fujimoto S, Horike H, Tokura T, Namikoshi T, et al. A novel free radical scavenger, edarabone, protects against cisplatin-induced acute renal damage in vitro and in vivo. J Pharmacol Exp Ther. 2003. 305:1183–1190.18. Antunes LM, Darin JD, Bianchi Nde L. Effects of the antioxidants curcumin or selenium on cisplatin-induced nephrotoxicity and lipid peroxidation in rats. Pharmacol Res. 2001. 43:145–150.19. Naziroglu M, Karaoglu A, Aksoy AO. Selenium and high dose vitamin E administration protects cisplatin-induced oxidative damage to renal, liver and lens tissues in rats. Toxicology. 2004. 195:221–230.20. Siu LL, Moore MJ. Use of mesna to prevent ifosfamide-induced urotoxicity. Support Care Cancer. 1998. 6:144–154.21. Ypsilantis P, Tentes I, Assimakopoulos SF, Kortsaris A, Scopa CD, Simopoulos C. Mesna ameliorates intestinal mucosa damage after ifosfamide administration in the rabbit at a dose-related manner. J Surg Res. 2004. 121:84–91.22. Yeh J, Kim BS, Peresie J. Protection against cisplatin-induced ovarian damage by the antioxidant sodium 2-mercaptoethanesulfonate (mesna) in female rats. Am J Obstet Gynecol. 2008. 198:463.e1–463.e7.23. Dale O, Mortensen B, Thommesen L, Hagen B. Cisplatin toxicity in the rat may be influenced by anaesthetic agents. Acta Anaesthesiol Scand. 2000. 44:770.24. Oktay K, Schenken RS, Nelson JF. Proliferating cell nuclear antigen marks the initiation of follicular growth in the rat. Biol Reprod. 1995. 53:295–301.25. Yeh J, Adashi E. Yen S, Jaffe R, Barbieri R, editors. The ovarian life cycle. Reproductive endocrinology. 1999. 4th ed. Philadelphia, PA: Saunders WB;153–190.26. Stubbs SA, Hardy K, Da Silva-Buttkus P, Stark J, Webber LJ, Flanagan AM, et al. Anti-mullerian hormone protein expression is reduced during the initial stages of follicle development in human polycystic ovaries. J Clin Endocrinol Metab. 2005. 90:5536–5543.27. Lee MM, Donahoe PK. Mullerian inhibiting substance: a gonadal hormone with multiple functions. Endocr Rev. 1993. 14:152–164.28. Gruijters MJ, Visser JA, Durlinger AL, Themmen AP. Anti-Mullerian hormone and its role in ovarian function. Mol Cell Endocrinol. 2003. 211:85–90.29. Visser JA, Themmen AP. Anti-Mullerian hormone and folliculogenesis. Mol Cell Endocrinol. 2005. 234:81–86.30. Themmen AP. Anti-Mullerian hormone: its role in follicular growth initiation and survival and as an ovarian reserve marker. J Natl Cancer Inst Monogr. 2005. 34:18–21.31. Streuli I, Fraisse T, Pillet C, Ibecheole V, Bischof P, de Ziegler D. Serum antimüllerian hormone levels remain stable throughout the menstrual cycle and after oral or vaginal administration of synthetic sex steroids. Fertil Steril. 2008. 90:395–400.32. Yeh J, Kim B, Liang YJ, Peresie J. Mullerian inhibiting substance as a novel biomarker of cisplatin-induced ovarian damage. Biochem Biophys Res Commun. 2006. 348:337–344.33. Visser JA, de Jong FH, Laven JS, Themmen AP. Anti-Mullerian hormone: a new marker for ovarian function. Reproduction. 2006. 131:1–9.34. Rosendahl M, Andersen CY, la Cour Freiesleben N, Juul A, Lossl K, Andersen AN. Dynamics and mechanisms of chemotherapy-induced ovarian follicular depletion in women of fertile age. Fertil Steril. 2010. 94:156–166.35. Chirino YI, Pedraza-Chaverri J. Role of oxidative and nitrosative stress in cisplatin-induced nephrotoxicity. Exp Toxicol Pathol. 2009. 61:223–242.36. Kabasakal L, Sehirli AO, Cetinel S, Cikler E, Gedik N, Sener G. Mesna (2-mercaptoethane sulfonate) prevents ischemia/reperfusion induced renal oxidative damage in rats. Life Sci. 2004. 75:2329–2340.37. Hausheer FH, Kanter P, Cao S, Haridas K, Seetharamulu P, Reddy D, et al. Modulation of platinum-induced toxicities and therapeutic index: mechanistic insights and first- and second-generation protecting agents. Semin Oncol. 1998. 25:584–599.38. Oprea A, Bazzazi H, Kangarloo B, Wolff JE. The kinetics and mechanisms of the reaction of Mesna with cisplatin, oxiplatin and carboplatin. Anticancer Res. 2001. 21:1225–1229.39. Kangarloo SB, Gangopadhyay SB, Syme RM, Wolff JE, Gluck S. Influence of mesna on the pharmacokinetics of cisplatin and carboplatin in pediatric cancer patients. Med Oncol. 2004. 21:9–20.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Efficacy of Ifosfamide-Based Regimen in Refractory of Relapsed Ovarian Cancer
- Fatal Ifosfamide-Induced Metabolic Encephalopathy in Patients with Recurrent Epithelial Ovarian Cancer: Report of Two Cases
- Effects and Mechanism of AP39 on Ovarian Functions in Rats Exposed to Cisplatin and Chronic Immobilization Stress
- Chronic Cyclophosphamide Induced Cystitis that is Improved by Mesna
- A New Rat Model of Cisplatin-induced Neuropathic Pain