J Clin Neurol.
2009 Dec;5(4):153-166. 10.3988/jcn.2009.5.4.153.
Current Challenges for the Early Detection of Alzheimer's Disease: Brain Imaging and CSF Studies
- Affiliations
-
- 1Department of Psychiatry, New York University School of Medicine, New York, NY, USA. lisa.mosconi@nyumc.org
- 2Nathan Kline Institute, Orangeburg, NY, USA.
- KMID: 2287644
- DOI: http://doi.org/10.3988/jcn.2009.5.4.153
Abstract
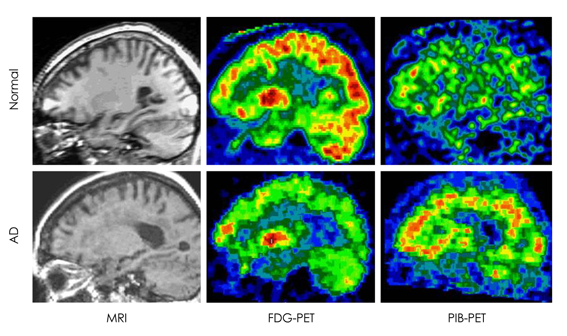
- The development of prevention therapies for Alzheimer's disease (AD) would greatly benefit from biomarkers that are sensitive to the subtle brain changes that occur in the preclinical stage of the disease. Reductions in the cerebral metabolic rate of glucose (CMRglc), a measure of neuronal function, have proven to be a promising tool in the early diagnosis of AD. In vivo brain 2-[18F]fluoro-2-Deoxy-D-glucose-positron emission tomography (FDG-PET) imaging demonstrates consistent and progressive CMRglc reductions in AD patients, the extent and topography of which correlate with symptom severity. There is increasing evidence that hypometabolism appears during the preclinical stages of AD and can predict decline years before the onset of symptoms. This review will give an overview of FDG-PET results in individuals at risk for developing dementia, including: presymptomatic individuals carrying mutations responsible for early-onset familial AD; patients with Mild Cognitive Impairment (MCI), often a prodrome to late-onset sporadic AD; non-demented carriers of the Apolipoprotein E (ApoE) epsilon4 allele, a strong genetic risk factor for late-onset AD; cognitively normal subjects with a family history of AD; subjects with subjective memory complaints; and normal elderly followed longitudinally until they expressed the clinical symptoms and received post-mortem confirmation of AD. Finally, we will discuss the potential to combine different PET tracers and CSF markers of pathology to improve the early detection of AD.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Measurement of Precuneal and Hippocampal Volumes Using Magnetic Resonance Volumetry in Alzheimer's Disease
Seon-Young Ryu, Min Jeong Kwon, Sang-Bong Lee, Dong Won Yang, Tae-Woo Kim, In-Uk Song, Po Song Yang, Hyun Jeong Kim, Ae Young Lee
J Clin Neurol. 2010;6(4):196-203. doi: 10.3988/jcn.2010.6.4.196.A Common Pathogenic Mechanism Linking Type-2 Diabetes and Alzheimer's Disease: Evidence from Animal Models
Sun Ah Park
J Clin Neurol. 2011;7(1):10-18. doi: 10.3988/jcn.2011.7.1.10.
Reference
-
1. Kung HC, Hoyert DL, Xu J, Murphy SL. Deaths: final data for 2005. Natl Vital Stat Rep. 2008. 56:1–120.2. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurol. 1984. 34:939–944.
Article3. Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch Neurol. 2003. 60:1119–1122.4. Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurol. 1991. 41:479–486.
Article5. Price JL, Morris JC. Tangles and plaques in nondemented aging and "preclinical" Alzheimer's disease. Ann Neurol. 1999. 45:358–368.
Article6. Braak H, Braak E. Development of Alzheimer-related neurofibrillary changes in the neocortex inversely recapitulates cortical myelogenesis. Acta Neuropathol. 1996. 92:197–201.
Article7. Delacourte A, David JP, Sergeant N, Buée L, Wattez A, Vermersch P, et al. The biochemical pathway of neurofibrillary degeneration in aging and Alzheimer's disease. Neurol. 1999. 52:1158–1165.
Article8. Morris JC, Storandt M, McKeel DW Jr, Rubin EH, Price JL, Grant EA, et al. Cerebral amyloid deposition and diffuse plaques in "normal" aging: Evidence for presymptomatic and very mild Alzheiemer's disease. Neurology. 1996. 46:707–719.
Article9. Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991. 82:239–259.
Article10. Morrison JH, Hof PR. Life and death of neurons in the aging brain. Science. 1997. 278:412–419.
Article11. Hyman BT, Van Hoesen GW, Damasio AR, Barnes CL. Alzheimer's disease: cell-specific pathology isolates the hippocampal formation. Science. 1984. 225:1168–1170.
Article12. Etiene D, Kraft J, Ganju N, Gomez-Isla T, Gemelli B, Hyman BT. Cerebrovascular Pathology Contributes to the Heterogeneity of Alzheimer's Disease. J Alzheimers Dis. 1998. 1:119–134.
Article13. Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR. Brain infarction and the clinical expression of Alzheimer's disease. The Nun Study. JAMA. 1997. 277:813–817.
Article14. Arriagada PV, Marzloff K, Hyman BT. Distribution of Alzheimer-type pathologic changes in nondemented elderly individuals matches the pattern in Alzheimer's disease. Neurology. 1992. 42:1681–1688.
Article15. Giannakopoulos P, Hof PR, Mottier S, Michel JP, Bouras C. Neuropathological changes in the cerebral cortex of 1258 cases from a geriatric hospital: retrospective clinicopathological evaluation of a 10-year autopsy population. Acta Neuropathol. 1994. 87:456–468.
Article16. Ulrich J. Alzheimer changes in nondemented patients younger than sixty-five: possible early stages of Alzheimer's disease and senile dementia of Alzheimer type. Ann Neurol. 1985. 17:273–277.
Article17. Selkoe DJ. Alzheimer's disease: genotypes, phenotype, and treatments. Science. 1997. 275:630–631.
Article18. Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M, et al. Diffusible, nonfibrillar ligands derived from Abeta1-42 are potent central nervous system neurotoxins. Proc Natl Acad Sci U S A. 1998. 95:6448–6453.
Article19. Consensus report of the Working Group on: "Molecular and Biochemical Markers of Alzheimer's Disease". The Ronald and Nancy Reagan Research Institute of the Alzheimers Association and the National Institute on Aging. Neurobiol Aging. 1998. 19:109–116.20. Nestor PJ, Scheltens P, Hodges JR. Advances in the early detection of Alzheimer's disease. Nat Med. 2004. 10:Suppl. S34–S41.
Article21. de Leon MJ, McRae T, Tsai JR, George AE, Marcus DL, Freedman M, et al. Abnormal cortisol response in Alzheimer's disease linked to hippocampal atrophy. Lancet. 1988. 2:391–392.
Article22. de Leon MJ, George AE, Stylopoulos LA, Smith G, Miller DC. Early marker for Alzheimer's disease: the atrophic hippocampus. Lancet. 1989. 2:672–673.
Article23. Jack CR Jr, Petersen RC, Xu Y, O'Brien PC, Smith GE, Ivnik RJ, et al. Rates of hippocampal atrophy correlate with change in clinical status in aging and AD. Neurology. 2000. 55:484–489.
Article24. Rusinek H, De Santi S, Frid D, Tsui W, Tarshish C, Convit A, et al. Regional brain atrophy rate predicts future cognitive decline: 6-year longitudinal MR imaging study of normal aging. Radiology. 2003. 229:691–696.
Article25. den Heijer T, Geerlings MI, Hoebeek FE, Hofman A, Koudstaal PJ, Breteler MM. Use of hippocampal and amygdalar volumes on magnetic resonance imaging to predict dementia in cognitively intact elderly people. Arch Gen Psychiatry. 2006. 63:57–62.
Article26. O'Sullivan M, Ngo E, Viswanathan A, Jouvent E, Gschwendtner A, Saemann PG, et al. Hippocampal volume is an independent predictor of cognitive performance in CADASIL. Neurobiol Aging. 2009. 30:890–897.27. Sokoloff L. Relation between physiological functions and energy metabolism in the central nervous system. J Neurochem. 1977. 29:13–26.
Article28. Magistretti PJ, Pellerin L, Rothman DL, Shulman RG. Energy on demand. Science. 1999. 283:496–497.
Article29. Attwell D, Iadecola C. The neural basis of functional brain imaging signals. Trends Neurosci. 2002. 25:621–625.
Article30. Malonek D, Grinvald A. Interactions between electrical activity and cortical microcirculation revealed by imaging spectroscopy: implications for functional brain mapping. Science. 1996. 272:551–554.
Article31. Rocher AB, Chapon F, Blaizot X, Baron JC, Chavoix C. Resting-state brain glucose utilization as measured by PET is directly related to regional synaptophysin levels: a study in baboons. Neuroimage. 2003. 20:1894–1898.
Article32. Magistretti PJ, Pellerin L. The contribution of astrocytes to the 18F-2-deoxyglucose signal in PET activation studies. Mol Psychiatry. 1996. 1:445–452.33. Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci U S A. 1994. 91:10625–10629.
Article34. Mosconi L. Brain glucose metabolism in the early and specific diagnosis of Alzheimer's disease. FDG-PET studies in MCI and AD. Eur J Nucl Med Mol Imaging. 2005. 32:486–510.
Article35. Friedland RP, Budinger TF, Ganz E, Yano Y, Mathis CA, Koss B, et al. Regional cerebral metabolic alterations in dementia of the Alzheimer type: positron emission tomography with [18F]fluorode-oxyglucose. J Comput Assist Tomogr. 1983. 7:590–598.
Article36. Frackowiak RS, Pozzilli C, Legg NJ, DuBoulay GH, Marshall J, Lenzi GL, et al. A prospective study of regional cerebral blood flow and oxygen utilization in dementia using positron emission tomography and oxygen-15. J Cereb Blood Flow Metab. 1981. 1:S453–S454.37. Minoshima S, Giordani B, Berent S, Frey KA, Foster NL, Kuhl DE. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer's disease. Ann Neurol. 1997. 42:85–94.
Article38. Ferris SH, de Leon MJ, Wolf AP, Farkas T, Christman DR, Reisberg B, et al. Positron emission tomography in the study of aging and senile dementia. Neurobiol Aging. 1980. 1:127–131.
Article39. Kim EJ, Cho SS, Jeong Y, Park KC, Kang SJ, Kang E, et al. Glucose metabolism in early onset versus late onset Alzheimers disease: an SPM analysis of 120 patients. Brain. 2005. 128:1790–1801.
Article40. Foster NL, Chase TN, Mansi L, Brooks R, Fedio P, Patronas NJ, et al. Cortical abnormalities in Alzheimer's disease. Ann Neurol. 1984. 16:649–654.
Article41. Mazziotta JC, Phelps ME. Phelps ME, Mazziotta JC, Schelbert H, editors. Positron Emission Tomography studies of the brain. Positron Emission Tomography & Autoradiography: Principles & Applications for the Brain & Heart. 1986. New York: Raven Press;493–579.42. Herholz K, Salmon E, Perani D, Baron JC, Holthoff V, Frölich L, et al. Discrimination between Alzheimer dementia and controls by automated analysis of multicenter FDG PET. Neuroimage. 2002. 17:302–316.
Article43. Silverman DH, Small GW, Chang CY, Lu CS, Kung De Aburto MA, Chen W, et al. Positron emission tomography in evaluation of dementia: Regional brain metabolism and long-term outcome. JAMA. 2001. 286:2120–2127.
Article44. Szelies B, Mielke R, Herholz K, Heiss WD. Quantitative topographical EEG compared to FDG PET for classification of vascular and degenerative dementia. Electroencephalogr Clin Neurophysiol. 1994. 91:131–139.
Article45. Tanzi RE, Bertram L. New frontiers in Alzheimer's disease genetics. Neuron. 2001. 32:181–184.
Article46. Kennedy AM, Newman SK, Frackowiak RS, Cunningham VJ, Roques P, Stevens J, et al. Chromosome 14 linked familial Alzheimer's disease. A clinico-pathological study of a single pedigree. Brain. 1995. 118:185–205.47. Kennedy AM, Frackowiak RS, Newman SK, Bloomfield PM, Seaward J, Roques P, et al. Deficits in cerebral glucose metabolism demonstrated by positron emission tomography in individuals at risk of familial Alzheimer's disease. Neurosci Lett. 1995. 186:17–20.
Article48. Mosconi L, Sorbi S, de Leon MJ, Li Y, Nacmias B, Myoung PS, et al. Hypometabolism exceeds atrophy in presymptomatic early-onset familial Alzheimer's disease. J Nucl Med. 2006. 47:1778–1786.49. de Leon MJ, Convit A, Wolf OT, Tarshish CY, DeSanti S, Rusinek H, et al. Prediction of cognitive decline in normal elderly subjects with 2-[(18)F]fluoro-2-deoxy-D-glucose/positron-emission tomography (FDG/PET). Proc Natl Acad Sci U S A. 2001. 98:10966–10971.
Article50. Mosconi L, De Santi S, Li J, Tsui WH, Li Y, Boppana M, et al. Hippocampal hypometabolism predicts cognitive decline from normal aging. Neurobiol Aging. 2008. 29:676–692.
Article51. Petersen RC, Stevens JC, Ganguli M, Tangalos EG, Cummings JL, DeKosky ST. Practice parameter: early detection of dementia: mild cognitive impiarment (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001. 56:1133–1142.
Article52. Gauthier S, Reisberg B, Zaudig M, Petersen RC, Ritchie K, Broich K, et al. Mild cognitive impairment. Lancet. 2006. 367:1262–1270.
Article53. Herholz K, Nordberg A, Salmon E, Perani D, Kessler J, Mielke R, et al. Impairment of neocortical metabolism predicts progression in Alzheimer's disease. Dement Geriatr Cogn Disord. 1999. 10:494–504.
Article54. Arnaiz E, Jelic V, Almkvist O, Wahlund LO, Winblad B, Valind S, et al. Impaired cerebral glucose metabolism and cognitive functioning predict deterioration in mild cognitive impairment. Neuroreport. 2001. 12:851–855.
Article55. De Santi S, de Leon MJ, Rusinek H, Convit A, Tarshish CY, Roche A, et al. Hippocampal formation glucose metabolism and volume losses in MCI and AD. Neurobiol Aging. 2001. 22:529–539.
Article56. Nestor PJ, Fryer TD, Smielewski P, Hodges JR. Limbic hypometabolism in Alzheimer's disease and mild cognitive impairment. Ann Neurol. 2003. 54:343–351.
Article57. Chételat G, Desgranges B, de la Sayette V, Viader F, Eustache F, Baron JC. Mild cognitive impairment: Can FDG-PET predict who is to rapidly convert to Alzheimer's disease? Neurology. 2003. 60:1374–1377.
Article58. Drzezga A, Lautenschlager N, Siebner H, Riemenschneider M, Willoch F, Minoshima S, et al. Cerebral metabolic changes accompanying conversion of mild cognitive impairment into Alzheimer's disease: a PET follow-up study. Eur J Nucl Med Mol Imaging. 2003. 30:1104–1113.
Article59. Mosconi L, Tsui WH, De Santi S, Li J, Rusinek H, Convit A, et al. Reduced hippocampal metabolism in MCI and AD: automated FDG-PET image analysis. Neurology. 2005. 64:1860–1867.
Article60. Drzezga A, Grimmer T, Riemenschneider M, Lautenschlager N, Siebner H, Alexopoulus P, et al. Prediction of individual clinical outcome in MCI by means of genetic assessment and (18)F-FDG PET. J Nucl Med. 2005. 46:1625–1632.61. Mosconi L, De Santi S, Li Y, Li J, Zhan J, Tsui WH, et al. Visual rating of medial temporal lobe metabolism in mild cognitive impairment and Alzheimer's disease using FDG-PET. Eur J Nucl Med Mol Imaging. 2006. 33:210–221.
Article62. Anchisi D, Borroni B, Franceschi M, Kerrouche N, Kalbe E, Beuthien-Beumann B, et al. Heterogeneity of brain glucose metaboism in mild cognitive impairment and clinical progression to Alzheimer disease. Arch Neurol. 2005. 62:1728–1733.
Article63. Haxby JV, Grady CL, Koss E, Horwitz B, Heston L, Schapiro M, et al. Longitudinal study of cerebral metabolic asymmetries and associated neuropsychological patterns in early dementia of the Alzheimer type. Arch Neurol. 1990. 47:753–760.
Article64. Reed BR, Jagust WJ, Seab JP, Ober BA. Memory and regional cerebral blood flow in mildly symptomatic Alzheimer's disease. Neurology. 1989. 39:1537–1539.
Article65. Berent S, Giordani B, Foster N, Minoshima S, Lajiness-O'Neill R, Koeppe R, et al. Neuropsychological function and cerebral glucose utilization in isolated memory impairment and Alzheimer's disease. J Psychiatr Res. 1999. 33:7–16.
Article66. Mosconi L, Perani D, Sorbi S, Herholz K, Nacmias B, Holthoff V, et al. MCI conversion to dementia and the APOE genotype: a prediction study with FDG-PET. Neurology. 2004. 63:2332–2340.67. Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999. 56:303–308.68. Jagust W, Gitcho A, Sun F, Kuczynski B, Mungas D, Haan M. Brain imaging evidence of preclinical Alzheimer's disease in normal aging. Ann Neurol. 2006. 59:673–681.
Article69. Skurk T, Alberti-Huber C, Herder C, Hauner H. Relationship between adipocyte size and adipokine expression and secretion. J Clin Endocrinol Metab. 2007. 92:1023–1033.
Article70. Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, et al. Gene dose of apolipoprotein E type 4 Allele and the risk of Alzheimer's disease in late onset families. Science. 1993. 261:921–923.
Article71. Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. 1997. 278:1349–1356.
Article72. Laws SM, Hone E, Gandy S, Martins RN. Expanding the association between the APOE gene and the risk of Alzheimer's disease: possible roles for APOE promoter polymorphisms and alterations in APOE transcription. J Neurochem. 2003. 84:1215–1236.
Article73. Small GW, Mazziotta JC, Collins MT, Baxter LR, Phelps ME, Mandelkern MA, et al. Apolipoprotein E type 4 allele and cerebral glucose metabolism in relatives at risk for familial Alzheimer disease. JAMA. 1995. 273:942–947.
Article74. Reiman EM, Caselli RJ, Yun LS, Chen K, Bandy D, Minoshima S, et al. Preclinical evidence of Alzheimer's disease in persons homozygous for the epsilon 4 allele for apolipoprotein E. N Engl J Med. 1996. 334:752–758.
Article75. Small GW, Ercoli LM, Silverman DH, Huang SC, Komo S, Bookheimer S, et al. Cerebral metabolic and cognitive decline in persons at genetic risk for Alzheimer's disease. Proc Natl Acad Sci U S A. 2000. 97:6037–6042.
Article76. Reiman EM, Caselli RJ, Chen K, Alexander GE, Bandy D, Frost J. Declining brain activity in cognitively normal apolipoprotein E epsilon 4 heterozygotes: A foundation for using positron emission tomography to efficiently test treatments to prevent Alzheimer's disease. Proc Natl Acad Sci U S A. 2001. 98:3334–3339.
Article77. Reiman EM, Chen K, Alexander GE, Caselli RJ, Bandy D, Osborne D, et al. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer's dementia. Proc Natl Acad Sci U S A. 2004. 101:284–289.
Article78. Reiman EM, Uecker A, Caselli RJ, Lewis S, Bandy D, de Leon MJ, et al. Hippocampal volumes in cognitively normal persons at genetic risk for Alzheimer's disease. Ann Neurol. 1998. 44:288–291.
Article79. Mosconi L, De Santi S, Brys M, Tsui WH, Pirraglia E, Glodzik-Sobanska L, et al. Hypometabolism and altered cerebrospinal fluid markers in normal apolipoprotein E E4 carriers with subjective memory complaints. Biol Psychiatry. 2008. 63:609–618.
Article80. Geerlings MI, Jonker C, Bouter LM, Adér HJ, Schmand B. Association between memory complaints and incident Alzheimer's disease in elderly people with normal baseline cognition. Am J Psychiatry. 1999. 156:531–537.81. Bobinski M, de Leon MJ, Convit A, De Santi S, Wegiel J, Tarshish CY, et al. MRI of entorhinal cortex in mild Alzheimer's disease. Lancet. 1999. 353:38–40.
Article82. Cupples LA, Farrer LA, Sadovnick AD, Relkin N, Whitehouse P, Green RC. Estimating risk curves for first-degree relatives of patients with Alzheimer's disease: the REVEAL study. Genet Med. 2004. 6:192–196.
Article83. Green RC, Cupples LA, Go R, Benke KS, Edeki T, Griffith PA, et al. Risk of dementia among white and African American relatives of patients with Alzheimer disease. JAMA. 2002. 287:329–336.
Article84. Silverman JM, Ciresi G, Smith CJ, Marin DB, Schnaider-Beeri M. Variability of familial risk of Alzheimer disease across the late life span. Arch Gen Psychiatry. 2005. 62:565–573.
Article85. Mosconi L, Brys M, Switalski R, Mistur R, Glodzik L, Pirraglia E, et al. Maternal family history of Alzheimer's disease predisposes to reduced brain glucose metabolism. Proc Natl Acad Sci U S A. 2007. 104:19067–19072.
Article86. Mosconi L, Mistur R, Switalski R, Brys M, Glodzik L, Rich K, et al. Declining brain glucose metabolism in normal individuals with a maternal history of Alzheimer disease. Neurology. 2009. 72:513–520.
Article87. Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006. 443:787–795.
Article88. Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, et al. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann Neurol. 2004. 55:306–319.
Article89. Rowe CC, Ng S, Ackermann U, Gong SJ, Pike K, Savage G, et al. Imaging beta-amyloid burden in aging and dementia. Neurology. 2007. 68:1718–1725.90. Kemppainen NM, Aalto S, Wilson IA, Någren K, Helin S, Brück A, et al. Voxel-based analysis of PET amyloid ligand [11C]PIB uptake in Alzheimer disease. Neurology. 2006. 67:1575–1580.
Article91. Pike KE, Savage G, Villemagne VL, Ng S, Moss SA, Maruff P, et al. Beta-amyloid imaging and memory in non-demented individuals: evidence for preclinical Alzheimer's disease. Brain. 2007. 130:2837–2844.
Article92. Mintun MA, Larossa GN, Sheline YI, Dence CS, Lee SY, Mach RH, et al. [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology. 2006. 67:446–452.
Article93. Fagan AM, Mintun MA, Mach RH, Lee SY, Dence CS, Shah AR, et al. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Abeta42 in humans. Ann Neurol. 2006. 59:512–519.
Article94. Klunk WE, Mathis CA, Price JC, Lopresti BJ, DeKosky ST. Two-year follow-up of amyloid deposition in patients with Alzheimer's disease. Brain. 2006. 129:2805–2807.
Article95. Drzezga A, Grimmer T, Henriksen G, Stangier I, Perneczky R, Diehl-Schmid J, et al. Imaging of amyloid plaques and cerebral glucose metabolism in semantic dementia and Alzheimer's disease. Neuroimage. 2008. 39:619–633.
Article96. Mathis CA, Wang Y, Klunk WE. Imaging beta-amyloid plaques and neurofibrillary tangles in the aging human brain. Curr Pharm Des. 2004. 10:1469–1492.
Article97. Lockhart A, Lamb JR, Osredkar T, Sue LI, Joyce JN, Ye L, et al. PIB is a non-specific imaging marker of amyloid-beta (Abeta) peptide-related cerebral amyloidosis. Brain. 2007. 130:2607–2615.
Article98. Agdeppa ED, Kepe V, Liu J, Flores-Torres S, Satyamurthy N, Petric A, et al. Binding characteristics of radiofluorinated 6-dialkylamino-2-naphthylethylidene derivatives as positron emission tomography imaging probes for beta-amyloid plaques in Alzheimer's disease. J Neurosci. 2001. 21:RC189.99. Small GW, Kepe V, Ercoli LM, Siddarth P, Bookheimer SY, Miller KJ, et al. PET of brain amyloid and tau in mild cognitive impairment. N Engl J Med. 2006. 355:2652–2663.
Article100. de Leon MJ, Mosconi L, Logan J. Seeing what Alzheimer saw. Nat Med. 2007. 13:129–131.
Article101. Powell MR, Smith GE, Knopman DS, Parisi JE, Boeve BF, Petersen RC, et al. Cognitive measures predict pathologic Alzheimer disease. Arch Neurol. 2006. 63:865–868.
Article102. Rowe CC, Ackermann U, Browne W, Mulligan R, Pike KL, O'Keefe G, et al. Imaging of amyloid beta in Alzheimer's disease with 18F-BAY94-9172, a novel PET tracer: proof of mechanism. Lancet Neurol. 2008. 7:129–135.
Article103. Mitchell A, Brindle N. CSF phosphorylated tau--does it constitute an accurate biological test for Alzheimer's disease? Int J Geriat Psychiatry. 2003. 18:407–411.
Article104. Brys M, Mosconi L, De Santi S, Rich KE, de Leon MJ. CSF biomarkers for mild cognitive impairment. J Aging Health. 2006. 2:111–121.105. Blennow K, Hampel H. CSF markers for incipient Alzheimer's disease. Lancet Neurol. 2003. 2:605–613.
Article106. Blennow K, de Leon MJ, Zetterberg H. Alzheimer's disease. Lancet Neurology. 2006. 368:387–403.
Article107. Arai H, Ishiguro K, Ohno H, Moriyama M, Itoh N, Okamura N, et al. CSF phosphorylated tau protein and mild cognitive impairment: a prospective study. Exp Neurol. 2000. 166:201–203.
Article108. Hansson O, Zetterberg H, Buchhave P, Londos E, Blennow K, Minthon L. Association between CSF biomarkers and incipient Alzheimer's disease in patients with mild cognitive impairment: a follow-up study. Lancet Neurol. 2006. 5:228–234.
Article109. Brys M, Pirraglia E, Rich K, Rolstad S, Mosconi L, Switalski R, et al. Prediction and longitudinal study of CSF biomarkers in mild cognitive impairment. Neurobiol Aging. 2009. 30:682–690.
Article110. Büerger née Buch K, Padberg F, Nolde T, Teipel SJ, Stübner S, Haslinger A, et al. Cerebrospinal fluid tau protein shows a better discrimination in young old (<70 years) than in old old patients with Alzheimer's disease compared with controls. Neurosci Lett. 1999. 277:21–24.
Article111. Hulstaert F, Blennow K, Ivanoiu A, Schoonderwaldt HC, Riemenschneider M, De Deyn PP, et al. Improved discrimination of AD patients using beta-amyloid (1-42) and tau levels in CSF. Neurology. 1999. 52:1555–1562.
Article112. Andreasen N, Minthon L, Davidsson P, Vanmechelen E, Vanderstichele H, Winblad B, et al. Evaluation of CSF-tau and CSF-Abeta42 as diagnostic markers for Alzheimer disease in clinical practice. Arch Neurol. 2001. 58:373–379.113. Glodzik-Sobanska L, Pirraglia E, Brys M, De Santi S, Mosconi L, Rich KE, et al. The effects of normal aging and ApoE genotype on the levels of CSF biomarkers for Alzheimer's disease. Neurobiol Aging. 2009. 30:672–681.
Article114. Arai H, Morikawa Y, Higuchi M, Matsui T, Clark CM, Miura M, et al. Cerebrospinal fluid tau levels in neurodegenerative diseases with distinct tau-related pathology. Biochem Biophys Res Commun. 1997. 236:262–264.
Article115. Mollenhauer B, Bibl M, Trenkwalder C, Stiens G, Cepek L, Steinacker P, et al. Follow-up investigations in cerebrospinal fluid of patients with dementia with Lewy bodies and Alzheimers disease. J Neural Transm. 2005. 112:933–948.
Article116. Hesse C, Rosengren L, Andreasen N, Davidsson P, Vanderstichele H, Vanmechelen E, et al. Transient increase in total tau but not phosphotau in human cerebrospinal fluid after acute stroke. Neurosci Lett. 2001. 297:187–190.
Article117. Buerger K, Zinkowski R, Teipel SJ, Tapiola T, Arai H, Blennow K, et al. Differential diagnosis of Alzheimer disease with cerebrospinal fluid levels of tau protein phosphorylated at threonine 231. Arch Neurol. 2002. 59:1267–1272.
Article118. Buerger K, Zinkowski R, Teipel SJ, Arai H, DeBernardis J, Kerkman D, et al. Differentiation of geriatric major depression from Alzheimer's disease with CSF tau protein phosphorylated at threonine 231. Am J Psychiatry. 2003. 160:376–379.
Article119. Buerger K, Otto M, Teipel SJ, Zinkowski R, Blennow K, DeBernardis J, et al. Dissociation between CSF total tau and tau protein phosphorylated at threonine 231 in Creutzfeldt-Jakob disease. Neurobiol Aging. 2006. 27:10–15.
Article120. Vanmechelen E, Vanderstichele H, Davidsson P, Van Kerschaver E, Van DerPerre B, Sjögren M, et al. Quantification of tau phosphory-lated at threonine 181 in human cerebrospinal fluid: a sandwich ELISA with a synthetic phosphopeptide for standardization. Neurosci Lett. 2000. 285:49–52.
Article121. Sjögren M, Davidsson P, Tullberg M, Minthon L, Wallin A, Wikkelso C, et al. Both total and phosphorylated tau are increased in Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2001. 70:624–630.
Article122. Ishiguro K, Ohno H, Arai H, Yamaguchi H, Urakami K, Park JM, et al. Phosphorylated tau in human cerebrospinal fluid is a diagnostic marker for Alzheimer's disease. Neurosci Lett. 1999. 270:91–94.
Article123. Parnetti L, Lanari A, Amici S, Gallai V, Vanmechelen E, Hulstaert F, et al. CSF phosphorylated tau is a possible marker for discriminating Alzheimer's disease from dementia with Lewy bodies. Phospho-Tau International Study Group. Neurol Sci. 2001. 22:77–78.
Article124. de Leon MJ, DeSanti S, Zinkowski R, Mehta PD, Pratico D, Segal S, et al. Longitudinal CSF and MRI biomarkers improve the diagnosis of mild cognitive impairment. Neurobiol Aging. 2006. 27:394–401.
Article125. de Leon MJ, Segal S, Tarshish CY, DeSanti S, Zinkowski R, Mehta PD, et al. Longitudinal cerebrospinal fluid tau load increases in mild cognitive impairment. Neurosci Lett. 2002. 333:183–186.
Article126. Andreasen N, Blennow K. CSF biomarkers for mild cognitive impairment and early Alzheimer's disease. Clin Neurol Neurosurg. 2005. 107:165–173.
Article127. Tapiola T, Pirttilä T, Mikkonen M, Mehta PD, Alafuzoff I, Koivisto K, et al. Three-year follow-up of cerebrospinal fluid tau, B-amyloid 42 and 40 concentrations in Alzheimer's disease. Neurosci Lett. 2000. 280:119–122.
Article128. Bading JR, Yamada S, Mackic JB, Kirkman L, Miller C, Calero M, et al. Brain clearance of Alzheimer's amyloid-beta40 in the squirrel monkey: a SPECT study in a primate model of cerebral amyloid angiopathy. J Drug Target. 2002. 10:359–368.
Article129. Silverberg GD, Levinthal E, Sullivan EV, Bloch DA, Chang SD, Leverenz J, et al. Assessment of low-flow CSF drainage as a treatment for AD: results of a randomized pilot study. Neurology. 2002. 59:1139–1145.
Article130. Silverberg GD, Heit G, Huhn S, Jaffe RA, Chang SD, Bronte-Stewart H, et al. The cerebrospinal fluid production rate is reduced in dementia of the Alzheimer's type. Neurology. 2001. 57:1763–1766.
Article131. Peskind ER, Li G, Shofer J, Quinn JF, Kaye JA, Clark CM, et al. Age and apolipoprotein E*4 allele effects on cerebrospinal fluid beta-amyloid 42 in adults with normal cognition. Arch Neurol. 2006. 63:936–939.
Article132. Fagan AM, Roe CM, Xiong C, Mintun MA, Morris JC, Holtzman DM. Cerebrospinal fluid tau/beta-amyloid(42) ratio as a prediction of cognitive decline in nondemented older adults. Arch Neurol. 2007. 64:343–349.
Article133. Fukuyama R, Mizuno T, Mori S, Nakajima K, Fushiki S, Yanagisawa K. Age-dependent change in the levels of Abeta40 and Abeta42 in cerebrospinal fluid from control subjects, and a decrease in the ratio of Abeta42 to Abeta40 level in cerebrospinal fluid from Alzheimer's disease patients. Eur Neurol. 2000. 43:155–160.
Article134. Shoji M, Kanai M, Matsubara E, Tomidokoro Y, Shizuka M, Ikeda Y, et al. The levels of cerebrospinal fluid Abeta40 and Abeta42(43) are regulated age-dependently. Neurobiol Aging. 2001. 22:209–215.
Article135. Kanai M, Matsubara E, Isoe K, Urakami K, Nakashima K, Arai H, et al. Longitudinal study of cerebrospinal fluid levels of tau, A beta1-40, and A beta1-42(43) in Alzheimer's disease: a study in Japan. Ann Neurol. 1998. 44:17–26.
Article136. Mehta PD, Pirttilä T, Mehta SP, Sersen EA, Aisen PS, Wisniewski HM. Plasma and cerebrospinal fluid levels of amyloid beta proteins 1-40 and 1-42 in Alzheimer disease. Arch Neurol. 2000. 57:100–105.
Article137. Markesbery WR. Oxidative stress hypothesis in Alzheimer's disease. Free Radic Biol Med. 1997. 23:134–147.
Article138. Montine TJ, Markesbery WR, Zackert W, Sanchez SC, Roberts LJ 2nd, Morrow JD. The magnitude of brain lipid peroxidation correlates with the extent of degeneration but not with density of neuritic plaques or neurofibrillary tangles or with APOE genotype in Alzheimer's disease patients. Am J Pathol. 1999. 155:863–868.
Article139. Montine TJ, Markesbery WR, Morrow JD, Roberts LJ 2nd. Cerebrospinal fluid F2-isoprostane levels are increased in Alzheimer's disease. Ann Neurol. 1998. 44:410–413.
Article140. Praticó D. F(2)-isoprostanes: sensitive and specific non-invasive indices of lipid peroxidation in vivo. Atherosclerosis. 1999. 147:1–10.
Article141. Praticó D, Lawson JA, Rokach J, Fitzgerald GA. The isoprostanes in biology and medicine. Trends Endocrinol Metab. 2001. 12:243–247.
Article142. Nunomura A, Perry G, Aliev G, Hirai K, Takeda A, Balraj EK, et al. Oxidative damage is the earliest event in Alzheimer disease. J Neuropathol Exp Neurol. 2001. 60:759–767.
Article143. Praticó D, Uryu K, Leight S, Trojanowski JQ, Lee VM. Increased lipid peroxidation precedes amyloid plaque formation in an animal model of Alzheimer amyloidosis. J Neurosci. 2001. 21:4183–4187.
Article144. Markesbery WR, Kryscio RJ, Lovell MA, Morrow JD. Lipid peroxidation is an early event in the brain in amnestic mild cognitive impairment. Ann Neurol. 2005. 58:730–735.
Article145. Montine TJ, Beal MF, Cudkowicz ME, O'Donnell H, Margolin RA, McFarland L, et al. Increased CSF F2-isoprostane concentration in probable AD. Neurology. 1999. 52:562–565.
Article146. Praticó D, Clark CM, Lee VM, Trojanowski JQ, Rokach J, Fitz-Gerald GA. Increased 8,12-iso-iPF2alpha-VI in Alzheimer's disease: correlation of a noninvasive index of lipid peroxidation with disease severity. Ann Neurol. 2000. 48:809–812.147. Praticó D, Clark CM, Liun F, Lee VY, Trojanowski JQ. Increase of brain oxidative stress in mild cognitive impairment: a possible predictor of Alzheimer disease. Arch Neurol. 2002. 59:972–976.148. de Leon MJ, Mosconi L, Li Y, De Santi S, Yao Y, Tsui WH, et al. Longitudinal CSF isoprostane and MRI atrophy in the progression to AD. J Neurol. 2007. 254:1666–1675.
Article149. Yao Y, Zhukareva V, Sung S, Clark CM, Rokach J, Lee VM, et al. Enhanced brain levels of 8,12-iso-iPF2alpha-VI differentiate AD from frontotemporal dementia. Neurology. 2003. 61:475–478.
Article150. Grossman M, Farmer J, Leight S, Work M, Moore P, Van Deerlin V, et al. Cerebrospinal fluid profile in frontotemporal dementia and Alzheimer's disease. Ann Neurol. 2005. 57:721–729.
Article151. Montine TJ, Kaye JA, Montine KS, McFarland L, Morrow JD, Quinn JF. Cerebrospinal fluid abeta42, tau, and f2-isoprostane concentrations in patients with Alzheimer disease, other dementias, and in age-matched controls. Arch Pathol Lab Med. 2001. 125:510–512.
Article152. Mosconi L, Brys M, Glodzik-Sobanska L, De Santi S, Rusinek H, de Leon MJ. Early detection of Alzheimer's disease using neuroimaging. Exp Gerontol. 2007. 42:129–138.
Article153. Alexander GE, Chen K, Pietrini P, Rapoport SI, Reiman EM. Longitudinal PET Evaluation of Cerebral Metabolic Decline in Dementia: A Potential Outcome Measure in Alzheimer's Disease Treatment Studies. Am J Psychiatry. 2002. 159:738–745.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Role of Functional Imaging Techniques in the Dementia
- Structural MR Imaging in the Diagnosis of Alzheimer's Disease and Other Neurodegenerative Dementia: Current Imaging Approach and Future Perspectives
- Harnessing Cerebrospinal Fluid Biomarkers in Clinical Trials for Treating Alzheimer's and Parkinson's Diseases: Potential and Challenges
- Early Diagnosis of Alzheimer's Disease Using Neuroimaging: Focus on Recent MRI and PET Studies
- Longitudinal Observation of Early-Onset Alzheimer’s Disease Dementia with Positive CSF Biomarkers/Negative Amyloid-β Positron-Emission Tomography