Drug-Induced Parkinsonism
- Affiliations
-
- 1Department of Neurology, Chung-Ang University College of Medicine, Seoul, Korea.
- 2Parkinson/Alzheimer Center, Department of Neurology, University of Ulsan College of Medicine, Seoul, Korea. sjchung@amc.seoul.kr
- KMID: 2287613
- DOI: http://doi.org/10.3988/jcn.2012.8.1.15
Abstract
- Drug-induced parkinsonism (DIP) is the second-most-common etiology of parkinsonism in the elderly after Parkinson's disease (PD). Many patients with DIP may be misdiagnosed with PD because the clinical features of these two conditions are indistinguishable. Moreover, neurological deficits in patients with DIP may be severe enough to affect daily activities and may persist for long periods of time after the cessation of drug taking. In addition to typical antipsychotics, DIP may be caused by gastrointestinal prokinetics, calcium channel blockers, atypical antipsychotics, and antiepileptic drugs. The clinical manifestations of DIP are classically described as bilateral and symmetric parkinsonism without tremor at rest. However, about half of DIP patients show asymmetrical parkinsonism and tremor at rest, making it difficult to differentiate DIP from PD. The pathophysiology of DIP is related to drug-induced changes in the basal ganglia motor circuit secondary to dopaminergic receptor blockade. Since these effects are limited to postsynaptic dopaminergic receptors, it is expected that presynaptic dopaminergic neurons in the striatum will be intact. Dopamine transporter (DAT) imaging is useful for diagnosing presynaptic parkinsonism. DAT uptake in the striatum is significantly decreased even in the early stage of PD, and this characteristic may help in differentiating PD from DIP. DIP may have a significant and longstanding effect on patients' daily lives, and so physicians should be cautious when prescribing dopaminergic receptor blockers and should monitor patients' neurological signs, especially for parkinsonism and other movement disorders.
Keyword
MeSH Terms
-
Aged
Anticonvulsants
Antipsychotic Agents
Basal Ganglia
Calcium Channel Blockers
Dopamine Plasma Membrane Transport Proteins
Dopaminergic Neurons
Humans
Movement Disorders
Organothiophosphorus Compounds
Parkinson Disease
Parkinsonian Disorders
Tremor
Anticonvulsants
Antipsychotic Agents
Calcium Channel Blockers
Dopamine Plasma Membrane Transport Proteins
Organothiophosphorus Compounds
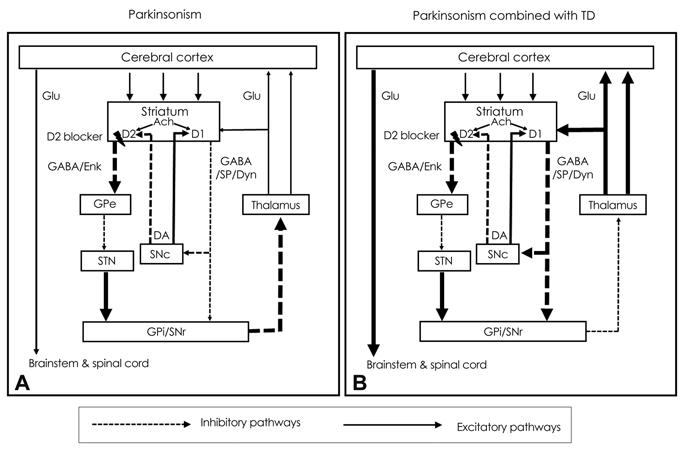
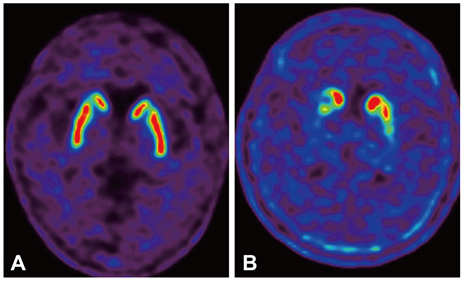
Figure
Cited by 5 articles
-
Clinical Perspectives of Parkinson's Disease for Ophthalmologists, Otorhinolaryngologists, Cardiologists, Dentists, Gastroenterologists, Urologists, Physiatrists, and Psychiatrists
Ji-Hyun Choi, Jong-Min Kim, Hee Kyung Yang, Hyo-Jung Lee, Cheol Min Shin, Seong Jin Jeong, Won-Seok Kim, Ji Won Han, In-Young Yoon, Yoo Sung Song, Yun Jung Bae
J Korean Med Sci. 2020;35(28):e230. doi: 10.3346/jkms.2020.35.e230.Cardiovascular Autonomic Dysfunction in Patients with Drug-Induced Parkinsonism
Joong-Seok Kim, Dong-Woo Ryu, Ju-Hee Oh, Yang-Hyun Lee, Sung-Jin Park, Kipyung Jeon, Jong-Yun Lee, Seong Hee Ho, Jungmin So, Jin Hee Im, Kwang-Soo Lee
J Clin Neurol. 2017;13(1):15-20. doi: 10.3988/jcn.2017.13.1.15.Trends in the Prevalence of Drug-Induced Parkinsonism in Korea
Ji-Hye Byun, Hyemin Cho, Yun Joong Kim, Joong-Seok Kim, Jong Sam Baik, Sunmee Jang, Hyeo-Il Ma
Yonsei Med J. 2019;60(8):760-767. doi: 10.3349/ymj.2019.60.8.760.The Correlation between Cognition Screening Scores and Gait Status from Three-Dimensional Gait Analysis
Jongki Choi, Jinse Park, Byung-Inn Lee, Kyoung jin Shin, Sunmi Yoo, Hyoeun Kim, Wooyoung Jang, Ji Sun Kim, Jinyoung Youn, Engseok Oh
J Clin Neurol. 2019;15(2):152-158. doi: 10.3988/jcn.2019.15.2.152.Combined Assessment of Serum Alpha-Synuclein and Rab35 is a Better Biomarker for Parkinson's Disease
Hung-Li Wang, Chin-Song Lu, Tu-Hsueh Yeh, Yu-Ming Shen, Yi-Hsin Weng, Ying-Zu Huang, Rou-Shayn Chen, Yu-Chuan Liu, Yi-Chuan Cheng, Hsiu-Chen Chang, Ying-Ling Chen, Yu-Jie Chen, Yan-Wei Lin, Chia-Chen Hsu, Huang-Li Lin, Chi-Han Chiu, Ching-Chi Chiu
J Clin Neurol. 2019;15(4):488-495. doi: 10.3988/jcn.2019.15.4.488.
Reference
-
1. Miller LG, Jankovic J. Neurologic approach to drug-induced movement disorders: a study of 125 patients. South Med J. 1990. 83:525–532.2. Montastruc JL, Llau ME, Rascol O, Senard JM. Drug-induced parkinsonism: a review. Fundam Clin Pharmacol. 1994. 8:293–306.
Article3. Sethi KD. Movement disorders induced by dopamine blocking agents. Semin Neurol. 2001. 21:59–68.
Article4. Esper CD, Factor SA. Failure of recognition of drug-induced parkinsonism in the elderly. Mov Disord. 2008. 23:401–404.
Article5. Kägi G, Bhatia KP, Tolosa E. The role of DAT-SPECT in movement disorders. J Neurol Neurosurg Psychiatry. 2010. 81:5–12.
Article6. Scherfler C, Schwarz J, Antonini A, Grosset D, Valldeoriola F, Marek K, et al. Role of DAT-SPECT in the diagnostic work up of parkinsonism. Mov Disord. 2007. 22:1229–1238.
Article7. Hall RA, Jackson RB, Swain JM. Neurotoxic reactions resulting from chlorpromazine administration. J Am Med Assoc. 1956. 161:214–218.
Article8. Benito-León J, Bermejo-Pareja F, Rodríguez J, Molina JA, Gabriel R, Morales JM. Neurological Disorders in Central Spain (NEDICES) Study Group. Prevalence of PD and other types of parkinsonism in three elderly populations of central Spain. Mov Disord. 2003. 18:267–274.
Article9. Wenning GK, Kiechl S, Seppi K, Müller J, Högl B, Saletu M, et al. Prevalence of movement disorders in men and women aged 50-89 years (Bruneck Study cohort): a population-based study. Lancet Neurol. 2005. 4:815–820.
Article10. Barbosa MT, Caramelli P, Maia DP, Cunningham MC, Guerra HL, Lima-Costa MF, et al. Parkinsonism and Parkinson's disease in the elderly: a community-based survey in Brazil (the Bambuí study). Mov Disord. 2006. 21:800–808.
Article11. Avorn J, Bohn RL, Mogun H, Gurwitz JH, Monane M, Everitt D, et al. Neuroleptic drug exposure and treatment of parkinsonism in the elderly: a case-control study. Am J Med. 1995. 99:48–54.
Article12. Susatia F, Fernandez HH. Drug-induced parkinsonism. Curr Treat Options Neurol. 2009. 11:162–169.
Article13. Thanvi B, Treadwell S. Drug induced parkinsonism: a common cause of parkinsonism in older people. Postgrad Med J. 2009. 85:322–326.
Article14. Volkow ND, Ding YS, Fowler JS, Wang GJ, Logan J, Gatley SJ, et al. Dopamine transporters decrease with age. J Nucl Med. 1996. 37:554–559.15. Stephen PJ, Williamson J. Drug-induced parkinsonism in the elderly. Lancet. 1984. 2:1082–1083.
Article16. Hardie RJ, Lees AJ. Neuroleptic-induced Parkinson's syndrome: clinical features and results of treatment with levodopa. J Neurol Neurosurg Psychiatry. 1988. 51:850–854.
Article17. Miller LG, Jankovic J. Metoclopramide-induced movement disorders. Clinical findings with a review of the literature. Arch Intern Med. 1989. 149:2486–2492.
Article18. Bedard P, Langelier P, Villeneuve A. Oestrogens and extrapyramidal system. Lancet. 1977. 2:1367–1368.19. Metzer WS, Newton JE, Steele RW, Claybrook M, Paige SR, McMillan DE, et al. HLA antigens in drug-induced parkinsonism. Mov Disord. 1989. 4:121–128.
Article20. Liou YJ, Wang YC, Chen JY, Bai YM, Lin CC, Liao DL, et al. Association analysis of polymorphisms in the N-methyl-D-aspartate (NMDA) receptor subunit 2B (GRIN2B) gene and tardive dyskinesia in schizophrenia. Psychiatry Res. 2007. 153:271–275.
Article21. Inada T, Koga M, Ishiguro H, Horiuchi Y, Syu A, Yoshio T, et al. Pathway-based association analysis of genome-wide screening data suggest that genes associated with the gamma-aminobutyric acid receptor signaling pathway are involved in neuroleptic-induced, treatment-resistant tardive dyskinesia. Pharmacogenet Genomics. 2008. 18:317–323.
Article22. Shanon J, Kaplan SM, Pierce CM, Ross WD. An interesting reaction to a tranquilizer: tonic seizures with perphenazine (trilafon). Am J Psychiatry. 1957. 114:556.
Article23. Christian CD, Paulson G. Severe motility disturbance after small doses of prochlorperazine. N Engl J Med. 1958. 259:828–830.
Article24. Janno S, Holi M, Tuisku K, Wahlbeck K. Prevalence of neuroleptic-induced movement disorders in chronic schizophrenia inpatients. Am J Psychiatry. 2004. 161:160–163.
Article25. Ossowska K. Neuronal basis of neuroleptic-induced extrapyramidal side effects. Pol J Pharmacol. 2002. 54:299–312.26. Tarsy D, Baldessarini RJ, Tarazi FI. Effects of newer antipsychotics on extrapyramidal function. CNS Drugs. 2002. 16:23–45.
Article27. Kuroki T, Nagao N, Nakahara T. Neuropharmacology of second-generation antipsychotic drugs: a validity of the serotonin-dopamine hypothesis. Prog Brain Res. 2008. 172:199–212.
Article28. Kapur S, Seeman P. Does fast dissociation from the dopamine d(2) receptor explain the action of atypical antipsychotics?: a new hypothesis. Am J Psychiatry. 2001. 158:360–369.
Article29. Stahl SM. "Hit-and-Run" actions at dopamine receptors, part 2: illustrating fast dissociation from dopamine receptors that typifies atypical antipsychotics. J Clin Psychiatry. 2001. 62:747–748.
Article30. The Parkinson Study Group. Low-dose clozapine for the treatment of drug-induced psychosis in Parkinson's disease. N Engl J Med. 1999. 340:757–763.31. Schotte A, Janssen PF, Gommeren W, Luyten WH, Van Gompel P, Lesage AS, et al. Risperidone compared with new and reference antipsychotic drugs: in vitro and in vivo receptor binding. Psychopharmacology (Berl). 1996. 124:57–73.
Article32. Gómez JC, Sacristán JA, Hernández J, Breier A, Ruiz Carrasco P, Antón Saiz C, et al. The safety of olanzapine compared with other antipsychotic drugs: results of an observational prospective study in patients with schizophrenia (EFESO Study). Pharmacoepidemiologic Study of Olanzapine in Schizophrenia. J Clin Psychiatry. 2000. 61:335–343.33. Rabey JM, Prokhorov T, Miniovitz A, Dobronevsky E, Klein C. Effect of quetiapine in psychotic Parkinson's disease patients: a double-blind labeled study of 3 months' duration. Mov Disord. 2007. 22:313–318.
Article34. Juncos JL, Roberts VJ, Evatt ML, Jewart RD, Wood CD, Potter LS, et al. Quetiapine improves psychotic symptoms and cognition in Parkinson's disease. Mov Disord. 2004. 19:29–35.
Article35. Wickremaratchi M, Morris HR, Ali IM. Aripiprazole associated with severe exacerbation of Parkinson's disease. Mov Disord. 2006. 21:1538–1539.
Article36. Sharma A, Sorrell JH. Aripiprazole-induced parkinsonism. Int Clin Psychopharmacol. 2006. 21:127–129.
Article37. Tonini M, Cipollina L, Poluzzi E, Crema F, Corazza GR, De Ponti F. Review article: clinical implications of enteric and central D2 receptor blockade by antidopaminergic gastrointestinal prokinetics. Aliment Pharmacol Ther. 2004. 19:379–390.
Article38. Kenney C, Hunter C, Davidson A, Jankovic J. Metoclopramide, an increasingly recognized cause of tardive dyskinesia. J Clin Pharmacol. 2008. 48:379–384.
Article39. Parkinsonism: an under-recognised complication of metoclopramide use. Ceylon Med J. 1996. 41:125.40. Sethi KD, Patel B, Meador KJ. Metoclopramide-induced parkinsonism. South Med J. 1989. 82:1581–1582.
Article41. Shin HW, Kim MJ, Kim JS, Lee MC, Chung SJ. Levosulpiride-induced movement disorders. Mov Disord. 2009. 24:2249–2253.
Article42. Madej TH. Domperidone--an acute dystonic reaction. Anaesthesia. 1985. 40:202.
Article43. Sørensen PS, Hansen K, Olesen J. A placebo-controlled, double-blind, cross-over trial of flunarizine in common migraine. Cephalalgia. 1986. 6:7–14.
Article44. García-Ruiz PJ, Javier Jiménez-Jiménez F, García de Yébenes J. Calcium channel blocker-induced parkinsonism: clinical features and comparisons with Parkinson's disease. Parkinsonism Relat Disord. 1998. 4:211–214.
Article45. Martí-Massó JF, Poza JJ. Cinnarizine-induced parkinsonism: ten years later. Mov Disord. 1998. 13:453–456.
Article46. Negrotti A, Calzetti S. A long-term follow-up study of cinnarizine- and flunarizine-induced parkinsonism. Mov Disord. 1997. 12:107–110.
Article47. Takada M, Kono T, Kitai ST. Flunarizine induces a transient loss of tyrosine hydroxylase immunoreactivity in nigrostriatal neurons. Brain Res. 1992. 590:311–315.
Article48. Onofrj M, Thomas A, Paci C. Reversible parkinsonism induced by prolonged treatment with valproate. J Neurol. 1998. 245:794–796.
Article49. Easterford K, Clough P, Kellett M, Fallon K, Duncan S. Reversible parkinsonism with normal beta-CIT-SPECT in patients exposed to sodium valproate. Neurology. 2004. 62:1435–1437.
Article50. Jamora D, Lim SH, Pan A, Tan L, Tan EK. Valproate-induced Parkinsonism in epilepsy patients. Mov Disord. 2007. 22:130–133.
Article51. Reches A, Tietler J, Lavy S. Parkinsonism due to lithium carbonate poisoning. Arch Neurol. 1981. 38:471.
Article52. Holroyd S, Smith D. Disabling parkinsonism due to lithium: a case report. J Geriatr Psychiatry Neurol. 1995. 8:118–119.
Article53. Tinazzi M, Antonini A, Bovi T, Pasquin I, Steinmayr M, Moretto G, et al. Clinical and [123I]FP-CIT SPET imaging follow-up in patients with drug-induced parkinsonism. J Neurol. 2009. 256:910–915.
Article54. Hassin-Baer S, Sirota P, Korczyn AD, Treves TA, Epstein B, Shabtai H, et al. Clinical characteristics of neuroleptic-induced parkinsonism. J Neural Transm. 2001. 108:1299–1308.
Article55. Jaber M, Robinson SW, Missale C, Caron MG. Dopamine receptors and brain function. Neuropharmacology. 1996. 35:1503–1519.
Article56. Farde L, Nordström AL, Wiesel FA, Pauli S, Halldin C, Sedvall G. Positron emission tomographic analysis of central D1 and D2 dopamine receptor occupancy in patients treated with classical neuroleptics and clozapine. Relation to extrapyramidal side effects. Arch Gen Psychiatry. 1992. 49:538–544.
Article57. Gunne LM, Andrén PE. An animal model for coexisting tardive dyskinesia and tardive parkinsonism: a glutamate hypothesis for tardive dyskinesia. Clin Neuropharmacol. 1993. 16:90–95.
Article58. Gershanik OS. Drug-induced parkinsonism in the aged. Recognition and prevention. Drugs Aging. 1994. 5:127–132.59. Jiménez-Jiménez FJ, Ortí-Pareja M, Ayuso-Peralta L, Gasalla T, Cabrera-Valdivia F, Vaquero A, et al. Drug-induced parkinsonism in a movement disorders unit: a four-year survey. Parkinsonism Relat Disord. 1996. 2:145–149.
Article60. Davis MR, Votaw JR, Bremner JD, Byas-Smith MG, Faber TL, Voll RJ, et al. Initial human PET imaging studies with the dopamine transporter ligand 18F-FECNT. J Nucl Med. 2003. 44:855–861.61. Varrone A, Halldin C. Molecular imaging of the dopamine transporter. J Nucl Med. 2010. 51:1331–1334.
Article62. Diaz-Corrales FJ, Sanz-Viedma S, Garcia-Solis D, Escobar-Delgado T, Mir P. Clinical features and 123I-FP-CIT SPECT imaging in drug-induced parkinsonism and Parkinson's disease. Eur J Nucl Med Mol Imaging. 2010. 37:556–564.
Article63. Lorberboym M, Treves TA, Melamed E, Lampl Y, Hellmann M, Djaldetti R. [123I]-FP/CIT SPECT imaging for distinguishing drug-induced parkinsonism from Parkinson's disease. Mov Disord. 2006. 21:510–514.
Article64. Tinazzi M, Ottaviani S, Isaias IU, Pasquin I, Steinmayr M, Vampini C, et al. [123I]FP-CIT SPET imaging in drug-induced Parkinsonism. Mov Disord. 2008. 23:1825–1829.
Article65. Poewe W, Scherfler C. Role of dopamine transporter imaging in investigation of parkinsonian syndromes in routine clinical practice. Mov Disord. 2003. 18:Suppl 7. S16–S21.
Article66. Lavalaye J, Linszen DH, Booij J, Dingemans PM, Reneman L, Habraken JB, et al. Dopamine transporter density in young patients with schizophrenia assessed with [123]FP-CIT SPECT. Schizophr Res. 2001. 47:59–67.
Article67. Tolosa E, Coelho M, Gallardo M. DAT imaging in drug-induced and psychogenic parkinsonism. Mov Disord. 2003. 18:Suppl 7. S28–S33.
Article68. Fann WE, Lake CR. Amantadine versus trihexyphenidyl in the treatment of neuroleptic-induced parkinsonism. Am J Psychiatry. 1976. 133:940–943.69. Jankovic J. Tardive syndromes and other drug-induced movement disorders. Clin Neuropharmacol. 1995. 18:197–214.
Article70. Rajput AH, Rozdilsky B, Hornykiewicz O, Shannak K, Lee T, Seeman P. Reversible drug-induced parkinsonism. Clinicopathologic study of two cases. Arch Neurol. 1982. 39:644–646.71. Bishnoi M, Chopra K, Kulkarni SK. Protective effect of adenosine reuptake inhibitors in haloperidol-induced orofacial dyskinesia and associated behavioural, biochemical and neurochemical changes. Pharmacology. 2007. 79:171–183.
Article72. Tsai G, Goff DC, Chang RW, Flood J, Baer L, Coyle JT. Markers of glutamatergic neurotransmission and oxidative stress associated with tardive dyskinesia. Am J Psychiatry. 1998. 155:1207–1213.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of ReVersible Parkinsonism Induced by Valproate in a Patient with Bipolar Disorder
- Levosulpiride-induced Parkinsonim
- Three Cases of Flunarizine-induced Parkinsinism
- Manganese induced parkinsonism: a case report
- Clinical Aspects of the Differential Diagnosis of Parkinson’s Disease and Parkinsonism