J Clin Neurol.
2014 Apr;10(2):119-124. 10.3988/jcn.2014.10.2.119.
Clinical and Electrophysiologic Responses to Acetylcholinesterase Inhibitors in MuSK-Antibody-Positive Myasthenia Gravis: Evidence for Cholinergic Neuromuscular Hyperactivity
- Affiliations
-
- 1Department of Neurology, Yonsei University College of Medicine, Seoul, Korea. kimsm@yuhs.ac
- KMID: 2287529
- DOI: http://doi.org/10.3988/jcn.2014.10.2.119
Abstract
- BACKGROUND AND PURPOSE
Patients with muscle-specific tyrosine kinase (MuSK) antibody (MuSK-Ab)-positive myasthenia gravis (MG) show distinct responses to acetylcholinesterase inhibitors (AChEIs). Although clinical responses to AChEIs in MuSK-Ab MG are reasonably well known, little is known about the electrophysiologic responses to AChEIs. We therefore investigated the clinical and electrophysiologic responses to AChEIs in MuSK-Ab-positive MG patients.
METHODS
We retrospectively reviewed the medical records and electrodiagnostic findings of 17 MG patients (10 MuSK-Ab-positive and 7 MuSK-Ab-negative patients) who underwent electrodiagnostic testing before and after a neostigmine test (NT).
RESULTS
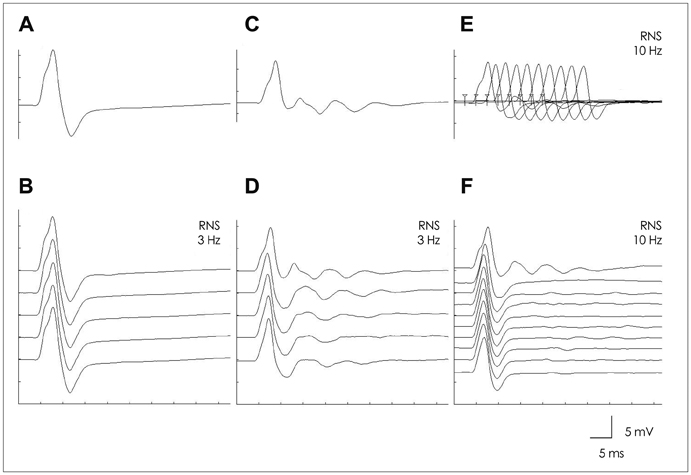
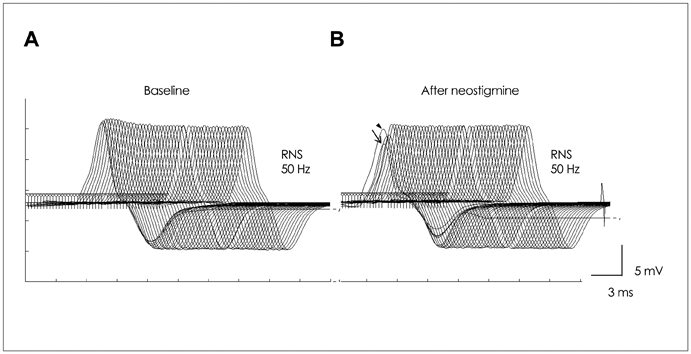
The frequency of intolerance to pyridostigmine bromide (PB) was higher in MuSK-Ab-positive patients than in MuSK-Ab-negative patients (50% vs. 0%, respectively; p=0.044), while the maximum tolerable dose of PB was lower in the former (90 mg/day vs. 480 mg/day, p=0.023). The frequency of positive NT results was significantly lower in MuSK-Ab-positive patients than in MuSK-Ab-negative patients (40% vs. 100%, p=0.035), while the nicotinic side effects of neostigmine were more frequent in the former (80% vs. 14.3%, p=0.015). Repetitive compound muscle action potentials (R-CMAPs) developed more frequently after NT in MuSK-Ab-positive patients than in MuSK-Ab-negative patients (90% vs. 14.3%, p=0.004). The frequency of a high-frequency-stimulation-induced decrement-increment pattern (DIP) was higher in MuSK-Ab-positive patients than in MuSK-Ab-negative patients (100% vs. 17.7%, p=0.003).
CONCLUSIONS
These results suggest that MuSK-Ab-positive MG patients exhibit unique and hyperactive responses to AChEIs. Furthermore, R-CMAP and DIP development on a standard AChEI dose may be a distinct neurophysiologic feature indicative of MuSK-Ab-positive MG.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Clinical Significance of Repetitive Compound Muscle Action Potentials in Patients with Myasthenia Gravis: A Predictor for Cholinergic Side Effects of Acetylcholinesterase Inhibitors
Hyo Eun Lee, Yool-hee Kim, Seung Min Kim, Ha Young Shin
J Clin Neurol. 2016;12(4):482-488. doi: 10.3988/jcn.2016.12.4.482.
Reference
-
1. Evoli A, Tonali PA, Padua L, Monaco ML, Scuderi F, Batocchi AP, et al. Clinical correlates with anti-MuSK antibodies in generalized seronegative myasthenia gravis. Brain. 2003; 126(Pt 10):2304–2311.
Article2. Guptill JT, Sanders DB, Evoli A. Anti-MuSK antibody myasthenia gravis: clinical findings and response to treatment in two large cohorts. Muscle Nerve. 2011; 44:36–40.
Article3. Rostedt Punga A, Ahlqvist K, Bartoccioni E, Scuderi F, Marino M, Suomalainen A, et al. Neurophysiological and mitochondrial abnormalities in MuSK antibody seropositive myasthenia gravis compared to other immunological subtypes. Clin Neurophysiol. 2006; 117:1434–1443.
Article4. Stickler DE, Massey JM, Sanders DB. MuSK-antibody positive myasthenia gravis: clinical and electrodiagnostic patterns. Clin Neurophysiol. 2005; 116:2065–2068.
Article5. Lavrnic D, Losen M, Vujic A, De Baets M, Hajdukovic LJ, Stojanovic V, et al. The features of myasthenia gravis with autoantibodies to MuSK. J Neurol Neurosurg Psychiatry. 2005; 76:1099–1102.
Article6. Punga AR, Flink R, Askmark H, Stålberg EV. Cholinergic neuromuscular hyperactivity in patients with myasthenia gravis seropositive for MuSK antibody. Muscle Nerve. 2006; 34:111–115.
Article7. van Dijk JG, Lammers GJ, Wintzen AR, Molenaar PC. Repetitive CMAPs: mechanisms of neural and synaptic genesis. Muscle Nerve. 1996; 19:1127–1133.
Article8. Jayawardane P, Senanayake N, Dawson A. Electrophysiological correlates of intermediate syndrome following acute organophosphate poisoning. Clin Toxicol (Phila). 2009; 47:193–205.
Article9. Kim JY, Park KD, Kim SM, Sunwoo IN. Decremental responses to repetitive nerve stimulation in x-linked bulbospinal muscular atrophy. J Clin Neurol. 2013; 9:32–35.
Article10. Moon JS, Sunwoo IN, Kim SM, Lee SA, Cho KH, Park KD, et al. Clinical analysis of 12 Korean Lambert-Eaton myasthenic syndrome (LEMS) patients. Yonsei Med J. 1999; 40:454–459.
Article11. Engel AG. Current status of the congenital myasthenic syndromes. Neuromuscul Disord. 2012; 22:99–111.
Article12. Cartaud A, Strochlic L, Guerra M, Blanchard B, Lambergeon M, Krejci E, et al. MuSK is required for anchoring acetylcholinesterase at the neuromuscular junction. J Cell Biol. 2004; 165:505–515.
Article13. Kawakami Y, Ito M, Hirayama M, Sahashi K, Ohkawara B, Masuda A, et al. Anti-MuSK autoantibodies block binding of collagen Q to MuSK. Neurology. 2011; 77:1819–1826.
Article14. Chroni E, Punga AR. Neurophysiological characteristics of MuSK antibody positive myasthenia gravis mice: focal denervation and hypersensitivity to acetylcholinesterase inhibitors. J Neurol Sci. 2012; 316:150–157.
Article15. Punga AR, Sawada M, Stålberg EV. Electrophysiological signs and the prevalence of adverse effects of acetylcholinesterase inhibitors in patients with myasthenia gravis. Muscle Nerve. 2008; 37:300–307.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Myasthenia Gravis in Pregnancy
- Clinical Significance of Repetitive Compound Muscle Action Potentials in Patients with Myasthenia Gravis: A Predictor for Cholinergic Side Effects of Acetylcholinesterase Inhibitors
- Muscle-Specific Receptor Tyrosine Kinase Antibody Positive Myasthenia Gravis Current Status
- Repetitive Nerve Stimulation in MuSK-Antibody-Positive Myasthenia Gravis
- Clinical Features of MuSK Antibody Positive Seronegative Myasthenia Gravis