J Clin Neurol.
2014 Oct;10(4):342-347. 10.3988/jcn.2014.10.4.342.
Recombinant Human Erythropoietin in Amyotrophic Lateral Sclerosis: A Pilot Study of Safety and Feasibility
- Affiliations
-
- 1Department of Neurology, College of Medicine, Hanyang University, Seoul, Korea. kimsh1@hanyang.ac.kr
- 2Bioengineering Institute, Corestem Inc., Seoul, Korea.
- KMID: 2287514
- DOI: http://doi.org/10.3988/jcn.2014.10.4.342
Abstract
- BACKGROUND AND PURPOSE
It has been shown that erythropoietin is neuroprotective in animal models of neurodegenerative diseases including amyotrophic lateral sclerosis (ALS). The aim of this study was to determine the safety and feasibility of repetitive high-dose recombinant human erythropoietin (rhEPO) therapy in ALS patients.
METHODS
Two consecutive studies were conducted. We first recruited 26 subjects for an initial single-arm safety study. After a lead-in period of 3 months to assess the disease progression, rhEPO was infused intravenously (35,000 IU) once per month for 3 months, and the subjects were followed for an additional 3 months. The ALS Functional Rating Scale-Revised (ALSFRS-R) was used for clinical assessment. After confirming the safety of rhEPO, 60 subjects were recruited for the second controlled study (rhEPO and control groups), which involved a total of 6 infusions at a rate of 1/month.
RESULTS
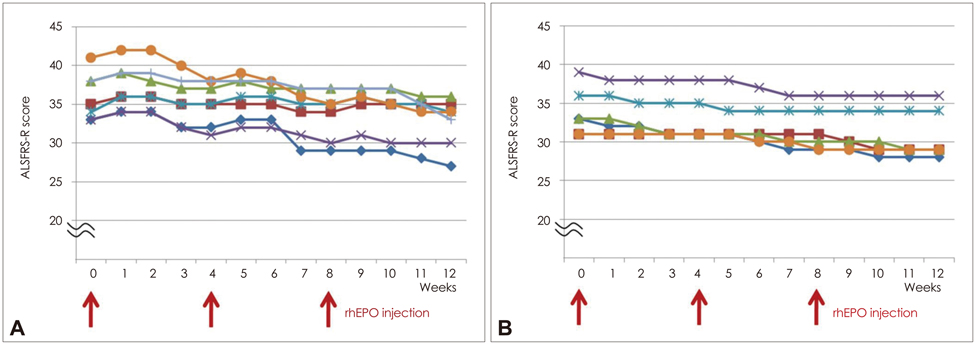
There were no serious adverse events in the first study. The mean rate of decline in the ALSFRS-R score was lower during the treatment period than during the lead-in period (mean+/-SD: 2.6+/-1.8 and 3.7+/-2.6, respectively; p=0.02). However, the rate of decline during the subsequent 3 months returned to that observed in the lead-in period. In the second study, the mean rate of decline in ALSFRS-R score was significantly lower in the rhEPO group than in the control group (during months 0-3, 1.8+/-1.7 vs. 3.1+/-2.3, p=0.03; during months 4-6, 2.1+/-2.2 vs. 3.5+/-2.3, p=0.02).
CONCLUSIONS
Intravenous high-dose rhEPO is both safe and feasible for the treatment of ALS. Further investigation using different intervals and doses should be considered.
MeSH Terms
Figure
Reference
-
1. Cleveland DW, Rothstein JD. From Charcot to Lou Gehrig: deciphering selective motor neuron death in ALS. Nat Rev Neurosci. 2001; 2:806–819.
Article2. Rowland LP, Shneider NA. Amyotrophic lateral sclerosis. N Engl J Med. 2001; 344:1688–1700.
Article3. de Carvalho M, Costa J, Swash M. Clinical trials in ALS: a review of the role of clinical and neurophysiological measurements. Amyotroph Lateral Scler Other Motor Neuron Disord. 2005; 6:202–212.
Article4. Bensimon G, Lacomblez L, Meininger V. ALS/Riluzole Study Group. A controlled trial of riluzole in amyotrophic lateral sclerosis. N Engl J Med. 1994; 330:585–591.
Article5. Miller RG, Mitchell JD, Lyon M, Moore DH. Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND). Amyotroph Lateral Scler Other Motor Neuron Disord. 2003; 4:191–206.
Article6. Assaraf MI, Diaz Z, Liberman A, Miller WH Jr, Arvanitakis Z, Li Y, et al. Brain erythropoietin receptor expression in Alzheimer disease and mild cognitive impairment. J Neuropathol Exp Neurol. 2007; 66:389–398.
Article7. Maiese K, Chong ZZ, Hou J, Shang YC. New strategies for Alzheimer's disease and cognitive impairment. Oxid Med Cell Longev. 2009; 2:279–289.8. Xue YQ, Zhao LR, Guo WP, Duan WM. Intrastriatal administration of erythropoietin protects dopaminergic neurons and improves neurobehavioral outcome in a rat model of Parkinson's disease. Neuroscience. 2007; 146:1245–1258.
Article9. Ehrenreich H, Fischer B, Norra C, Schellenberger F, Stender N, Stiefel M, et al. Exploring recombinant human erythropoietin in chronic progressive multiple sclerosis. Brain. 2007; 130(Pt 10):2577–2588.
Article10. Akdemir Ozisik P, Oruckaptan H, Ozdemir Geyik P, Misirlioglu M, Sargon MF, Kilinc K, et al. Effect of erythropoietin on brain tissue after experimental head trauma in rats. Surg Neurol. 2007; 68:547–555. discussion 555.
Article11. Ehrenreich H, Hinze-Selch D, Stawicki S, Aust C, Knolle-Veentjer S, Wilms S, et al. Improvement of cognitive functions in chronic schizophrenic patients by recombinant human erythropoietin. Mol Psychiatry. 2007; 12:206–220.
Article12. Krebs M, Leopold K, Hinzpeter A, Schaefer M. Neuroprotective agents in schizophrenia and affective disorders. Expert Opin Pharmacother. 2006; 7:837–848.
Article13. Koh SH, Kim Y, Kim HY, Cho GW, Kim KS, Kim SH. Recombinant human erythropoietin suppresses symptom onset and progression of G93A-SOD1 mouse model of ALS by preventing motor neuron death and inflammation. Eur J Neurosci. 2007; 25:1923–1930.
Article14. Konishi Y, Chui DH, Hirose H, Kunishita T, Tabira T. Trophic effect of erythropoietin and other hematopoietic factors on central cholinergic neurons in vitro and in vivo. Brain Res. 1993; 609:29–35.
Article15. Ehrenreich H, Aust C, Krampe H, Jahn H, Jacob S, Herrmann M, et al. Erythropoietin: novel approaches to neuroprotection in human brain disease. Metab Brain Dis. 2004; 19:195–206.
Article16. Janik P, Kwiecinski H, Sokolowska B, Niebroj-Dobosz I. Erythropoietin concentration in serum and cerebrospinal fluid of patients with amyotrophic lateral sclerosis. J Neural Transm. 2010; 117:343–347.
Article17. Ehrenreich H, Hasselblatt M, Dembowski C, Cepek L, Lewczuk P, Stiefel M, et al. Erythropoietin therapy for acute stroke is both safe and beneficial. Mol Med. 2002; 8:495–505.
Article18. Lipsic E, van der Meer P, Voors AA, Westenbrink BD, van den Heuvel AF, de Boer HC, et al. A single bolus of a long-acting erythropoietin analogue darbepoetin alfa in patients with acute myocardial infarction: a randomized feasibility and safety study. Cardiovasc Drugs Ther. 2006; 20:135–141.
Article19. Brooks BR, Miller RG, Swash M, Munsat TL. World Federation of Neurology Research Group on Motor Neuron Diseases. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000; 1:293–299.
Article20. Henry DH, Bowers P, Romano MT, Provenzano R. Epoetin alfa. Clinical evolution of a pleiotropic cytokine. Arch Intern Med. 2004; 164:262–276.21. Stohlawetz PJ, Dzirlo L, Hergovich N, Lackner E, Mensik C, Eichler HG, et al. Effects of erythropoietin on platelet reactivity and thrombopoiesis in humans. Blood. 2000; 95:2983–2989.
Article22. Maschio G. Erythropoietin and systemic hypertension. Nephrol Dial Transplant. 1995; 10:Suppl 2. 74–79.23. Smith KJ, Bleyer AJ, Little WC, Sane DC. The cardiovascular effects of erythropoietin. Cardiovasc Res. 2003; 59:538–548.
Article24. Cheung WK, Goon BL, Guilfoyle MC, Wacholtz MC. Pharmacokinetics and pharmacodynamics of recombinant human erythropoietin after single and multiple subcutaneous doses to healthy subjects. Clin Pharmacol Ther. 1998; 64:412–423.
Article25. Katavetin P, Inagi R, Miyata T, Shao J, Sassa R, Adler S, et al. Erythropoietin induces heme oxygenase-1 expression and attenuates oxidative stress. Biochem Biophys Res Commun. 2007; 359:928–934.
Article26. Villa P, Bigini P, Mennini T, Agnello D, Laragione T, Cagnotto A, et al. Erythropoietin selectively attenuates cytokine production and inflammation in cerebral ischemia by targeting neuronal apoptosis. J Exp Med. 2003; 198:971–975.
Article27. Chong ZZ, Kang JQ, Maiese K. Erythropoietin: cytoprotection in vascular and neuronal cells. Curr Drug Targets Cardiovasc Haematol Disord. 2003; 3:141–154.
Article28. Agnello D, Bigini P, Villa P, Mennini T, Cerami A, Brines ML, et al. Erythropoietin exerts an anti-inflammatory effect on the CNS in a model of experimental autoimmune encephalomyelitis. Brain Res. 2002; 952:128–134.
Article29. Cho GW, Koh SH, Kim MH, Yoo AR, Noh MY, Oh S, et al. The neuroprotective effect of erythropoietin-transduced human mesenchymal stromal cells in an animal model of ischemic stroke. Brain Res. 2010; 1353:1–13.
Article30. Genc S, Akhisaroglu M, Kuralay F, Genc K. Erythropoietin restores glutathione peroxidase activity in 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine-induced neurotoxicity in C57BL mice and stimulates murine astroglial glutathione peroxidase production in vitro. Neurosci Lett. 2002; 321:73–76.
Article31. Grignaschi G, Zennaro E, Tortarolo M, Calvaresi N, Bendotti C. Erythropoietin does not preserve motor neurons in a mouse model of familial ALS. Amyotroph Lateral Scler. 2007; 8:31–35.
Article32. Lauria G, Campanella A, Filippini G, Martini A, Penza P, Maggi L, et al. Erythropoietin in amyotrophic lateral sclerosis: a pilot, randomized, double-blind, placebo-controlled study of safety and tolerability. Amyotroph Lateral Scler. 2009; 10:410–415.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Amyotrophic Lateral Sclerosis Associated With CADASIL
- Syndrome of Progressive Bulbar Palsy in Amyotrophic Lateral Sclerosis: A Case Report
- Apraxia of Eyelid Closure and Motor Impersistence of Eyelid in a Patient with Amyotrophic Lateral Sclerosis
- Psychosocial Responses and Quality of Life among Amyotrophic Lateral Sclerosis Patients and Their Caregivers
- Diagnosis and management of amyotrophic lateral sclerosis