J Breast Cancer.
2015 Mar;18(1):22-28. 10.4048/jbc.2015.18.1.22.
Sentinel Lymph Node Biopsy Alone after Neoadjuvant Chemotherapy in Patients with Initial Cytology-Proven Axillary Node Metastasis
- Affiliations
-
- 1Department of Surgery, Ajou University School of Medicine, Suwon, Korea. smartblade@gmail.com
- 2MD Hospital, Seoul, Korea.
- 3Department of Surgery, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 4Ulsan City Hospital, Ulsan, Korea.
- KMID: 2286333
- DOI: http://doi.org/10.4048/jbc.2015.18.1.22
Abstract
- PURPOSE
Neoadjuvant chemotherapy (NAC) has been recently used to downstage breast cancer. However, in patients with initial axillary lymph node (ALN) metastasis, ALN dissection regardless of the NAC response remains the standard treatment. The purpose of this study was to identify the feasibility and accuracy of sentinel lymph node biopsy (SLNB) after NAC in patients with ALN metastasis at diagnosis.
METHODS
From January 2007 to August 2013, data of patients who were diagnosed with invasive breast cancer and ALN metastasis and treated with NAC followed by definitive surgery in two centers were collected retrospectively. A total of 386 patients were enrolled and classified into five groups according to surgical procedure for the ALNs and pathologic results.
RESULTS
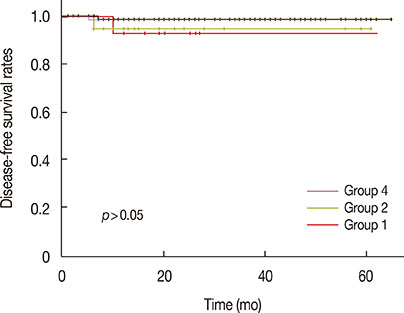
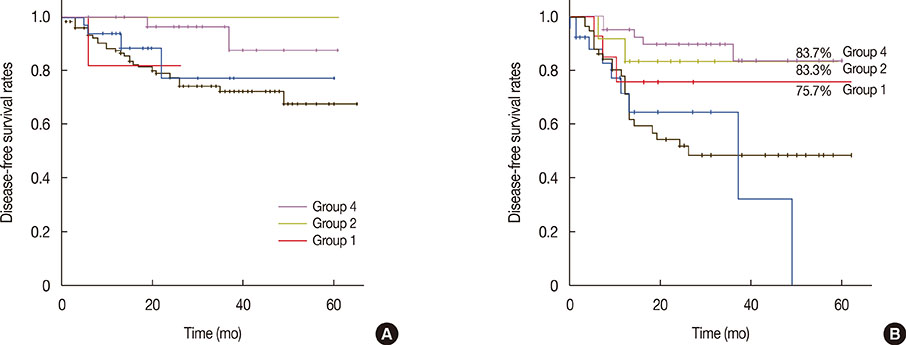
At SLNB after NAC, sentinel lymph nodes (SLNs) that stained blue or were hot, including suspicious nodes, were identified; the SLN identification and false-negative rates was 96% and 10%, respectively. There was no difference in the overall survival among the groups. For patients who revealed a pathologic complete node response, there was a significant difference in the disease-free survival rate between the SLNB only and complete ALN dissection groups (p=0.031). However, the rate of axillary recurrence demonstrated no significant differences among the groups (p>0.050).
CONCLUSION
SLNB after NAC in breast cancer patients with initial ALN metastasis may help identify downstaging to negative nodal status and thereby reduce the surgical morbidity by avoiding standard ALN dissection.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Use of Sentinel Lymph Node Biopsy after Neoadjuvant Chemotherapy in Patients with Axillary Node-Positive Breast Cancer in Diagnosis
Hee Jun Choi, Isaac Kim, Emad Alsharif, Sungmin Park, Jae-Myung Kim, Jai Min Ryu, Seok Jin Nam, Seok Won Kim, Jonghan Yu, Se Kyung Lee, Jeong Eon Lee
J Breast Cancer. 2018;21(4):433-441. doi: 10.4048/jbc.2018.21.e54.Targeted axillary biopsy and sentinel lymph node biopsy for axillary restaging after neoadjuvant chemotherapy
Gunay Gurleyik, Sibel Aydin Aksu, Fügen Aker, Kubra Kaytaz Tekyol, Eda Tanrikulu, Emin Gurleyik
Ann Surg Treat Res. 2021;100(6):305-312. doi: 10.4174/astr.2021.100.6.305.
Reference
-
1. Veronesi U, Paganelli G, Viale G, Luini A, Zurrida S, Galimberti V, et al. A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. N Engl J Med. 2003; 349:546–553.
Article2. Chen AM, Meric-Bernstam F, Hunt KK, Thames HD, Oswald MJ, Outlaw ED, et al. Breast conservation after neoadjuvant chemotherapy: the MD Anderson Cancer Center experience. J Clin Oncol. 2004; 22:2303–2312.
Article3. Loibl S, von Minckwitz G, Raab G, Blohmer JU, Dan Costa S, Gerber B, et al. Surgical procedures after neoadjuvant chemotherapy in operable breast cancer: results of the GEPARDUO trial. Ann Surg Oncol. 2006; 13:1434–1442.
Article4. Kuerer HM, Sahin AA, Hunt KK, Newman LA, Breslin TM, Ames FC, et al. Incidence and impact of documented eradication of breast cancer axillary lymph node metastases before surgery in patients treated with neoadjuvant chemotherapy. Ann Surg. 1999; 230:72–78.
Article5. Kuerer HM, Newman LA, Fornage BD, Dhingra K, Hunt KK, Buzdar AU, et al. Role of axillary lymph node dissection after tumor downstaging with induction chemotherapy for locally advanced breast cancer. Ann Surg Oncol. 1998; 5:673–680.
Article6. Vlastos G, Mirza NQ, Lenert JT, Hunt KK, Ames FC, Feig BW, et al. The feasibility of minimally invasive surgery for stage IIA, IIB, and IIIA breast carcinoma patients after tumor downstaging with induction chemotherapy. Cancer. 2000; 88:1417–1424.
Article7. Lyman GH, Giuliano AE, Somerfield MR, Benson AB 3rd, Bodurka DC, Burstein HJ, et al. American Society of Clinical Oncology guideline recommendations for sentinel lymph node biopsy in early-stage breast cancer. J Clin Oncol. 2005; 23:7703–7720.
Article8. von Minckwitz G, Kümmel S, Vogel P, Hanusch C, Eidtmann H, Hilfrich J, et al. Intensified neoadjuvant chemotherapy in early-responding breast cancer: phase III randomized GeparTrio study. J Natl Cancer Inst. 2008; 100:552–562.
Article9. von Minckwitz G, Blohmer JU, Raab G, Löhr A, Gerber B, Heinrich G, et al. In vivo chemosensitivity-adapted preoperative chemotherapy in patients with early-stage breast cancer: the GEPARTRIO pilot study. Ann Oncol. 2005; 16:56–63.
Article10. von Minckwitz G, Untch M, Blohmer JU, Costa SD, Eidtmann H, Fasching PA, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012; 30:1796–1804.
Article11. Yoo C, Ahn JH, Jung KH, Kim SB, Kim HH, Shin HJ, et al. Impact of immunohistochemistry-based molecular subtype on chemosensitivity and survival in patients with breast cancer following neoadjuvant chemotherapy. J Breast Cancer. 2012; 15:203–210.
Article12. Rouzier R, Perou CM, Symmans WF, Ibrahim N, Cristofanilli M, Anderson K, et al. Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin Cancer Res. 2005; 11:5678–5685.
Article13. Bhargava R, Beriwal S, Dabbs DJ, Ozbek U, Soran A, Johnson RR, et al. Immunohistochemical surrogate markers of breast cancer molecular classes predicts response to neoadjuvant chemotherapy: a single institutional experience with 359 cases. Cancer. 2010; 116:1431–1439.
Article14. Fisher B, Brown A, Mamounas E, Wieand S, Robidoux A, Margolese RG, et al. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol. 1997; 15:2483–2493.
Article15. Mamounas EP. Sentinel lymph node biopsy after neoadjuvant systemic therapy. Surg Clin North Am. 2003; 83:931–942.
Article16. Rouzier R, Extra JM, Klijanienko J, Falcou MC, Asselain B, Vincent-Salomon A, et al. Incidence and prognostic significance of complete axillary downstaging after primary chemotherapy in breast cancer patients with T1 to T3 tumors and cytologically proven axillary metastatic lymph nodes. J Clin Oncol. 2002; 20:1304–1310.
Article17. Charfare H, Limongelli S, Purushotham AD. Neoadjuvant chemotherapy in breast cancer. Br J Surg. 2005; 92:14–23.
Article18. Nason KS, Anderson BO, Byrd DR, Dunnwald LK, Eary JF, Mankoff DA, et al. Increased false negative sentinel node biopsy rates after preoperative chemotherapy for invasive breast carcinoma. Cancer. 2000; 89:2187–2194.
Article19. Pecha V, Kolarik D, Kozevnikova R, Hovorkova K, Hrabetova P, Halaska M, et al. Sentinel lymph node biopsy in breast cancer patients treated with neoadjuvant chemotherapy. Cancer. 2011; 117:4606–4616.
Article20. Gimbergues P, Abrial C, Durando X, Le Bouedec G, Cachin F, Penault-Llorca F, et al. Sentinel lymph node biopsy after neoadjuvant chemotherapy is accurate in breast cancer patients with a clinically negative axillary nodal status at presentation. Ann Surg Oncol. 2008; 15:1316–1321.
Article21. Mamounas EP, Brown A, Anderson S, Smith R, Julian T, Miller B, et al. Sentinel node biopsy after neoadjuvant chemotherapy in breast cancer: results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2005; 23:2694–2702.
Article22. Xing Y, Foy M, Cox DD, Kuerer HM, Hunt KK, Cormier JN. Metaanalysis of sentinel lymph node biopsy after preoperative chemotherapy in patients with breast cancer. Br J Surg. 2006; 93:539–546.
Article23. Boughey JC, Suman VJ, Mittendorf EA, Ahrendt GM, Wilke LG, Taback B, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA. 2013; 310:1455–1461.
Article24. Straver ME, Rutgers EJ, Russell NS, Oldenburg HS, Rodenhuis S, Wesseling J, et al. Towards rational axillary treatment in relation to neoadjuvant therapy in breast cancer. Eur J Cancer. 2009; 45:2284–2292.
Article25. Fisher B, Jeong JH, Anderson S, Bryant J, Fisher ER, Wolmark N. Twenty-five-year follow-up of a randomized trial comparing radical mastectomy, total mastectomy, and total mastectomy followed by irradiation. N Engl J Med. 2002; 347:567–575.
Article26. Bilimoria KY, Bentrem DJ, Hansen NM, Bethke KP, Rademaker AW, Ko CY, et al. Comparison of sentinel lymph node biopsy alone and completion axillary lymph node dissection for node-positive breast cancer. J Clin Oncol. 2009; 27:2946–2953.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Sentinel Lymph Node Biopsy in Breast Cancer: A Clinical Review and Update
- Use of Sentinel Lymph Node Biopsy after Neoadjuvant Chemotherapy in Patients with Axillary Node-Positive Breast Cancer in Diagnosis
- Evaluation of the Role of Axillary Lymph Node Fine-Needle Aspiration Cytology in Early Breast Cancer With or Without Neoadjuvant Chemotherapy
- Sentinel Lymph Node Biopsy in Patients with Clinically Negative Lymph Node After Neoadjuvant Chemotherapy
- Validation and Controversy of Sentinel Node Biopsy for Breast Cancer