Korean J Physiol Pharmacol.
2015 Jul;19(4):357-363. 10.4196/kjpp.2015.19.4.357.
Effect of Bevacizumab on Human Tenon's Fibroblasts Cultured from Primary and Recurrent Pterygium
- Affiliations
-
- 1Department of Ophthalmology, Pusan National University School of Medicine & Medical Research Institute, Yangsan Pusan National University Hospital, Yangsan 626-770, Korea.
- 2Department of Pharmacology, Pusan National University College of Medicine, and MRC for Ischemic Tissue Regeneration, Yangsan 626-870, Korea.
- 3Department of Ophthalmology, School of Medicine, Pusan National University & Medical Research Institute, Pusan National University Hospital, Pusan 602-739, Korea. jongsooluw@gmail.com
- KMID: 2285579
- DOI: http://doi.org/10.4196/kjpp.2015.19.4.357
Abstract
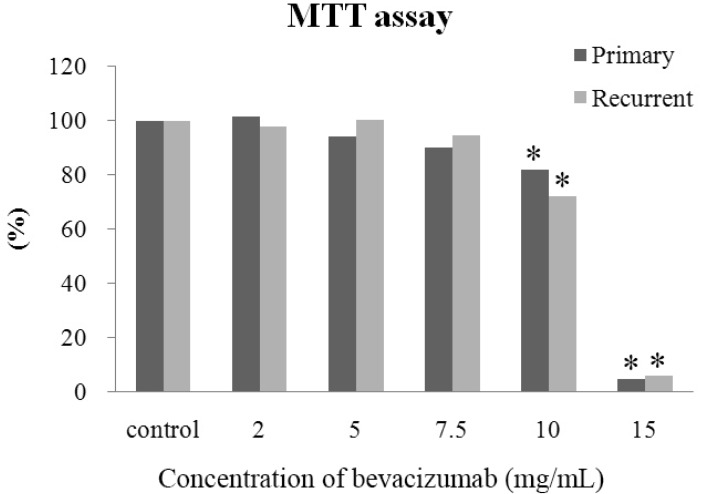
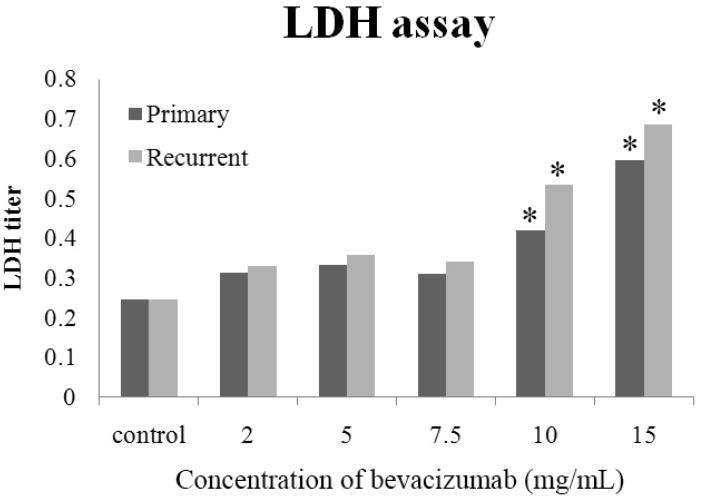
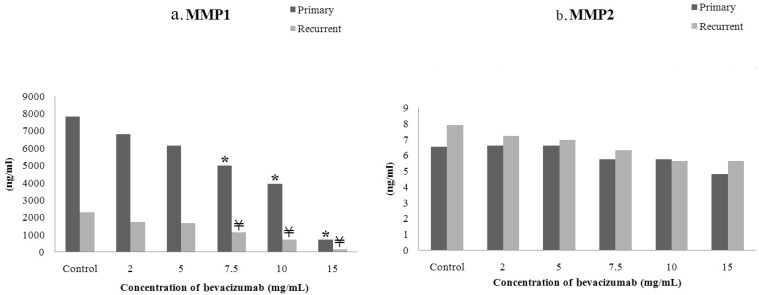
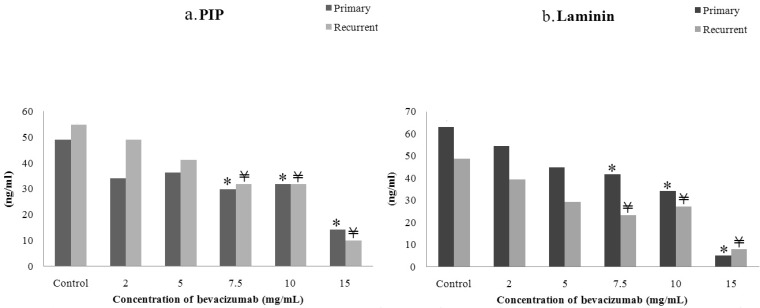
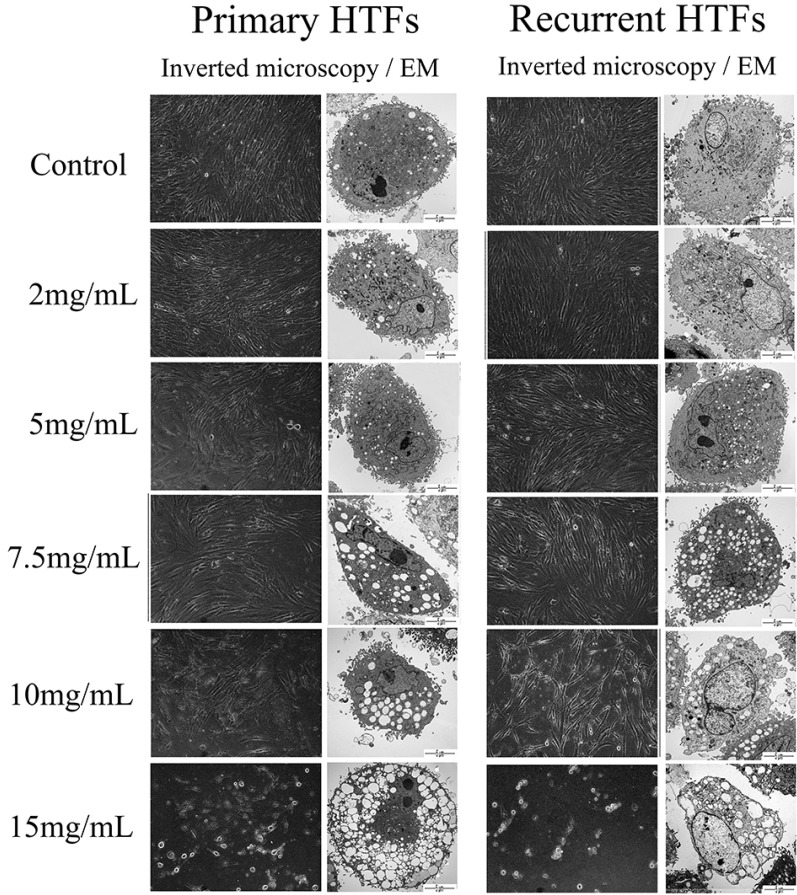
- The purpose of this study was to compare the inhibitory effect of bevacizumab on human Tenon's fibroblasts (HTFs) cultured from primary and recurrent pterygium. Cultured HTFs were exposed to 2.0, 5.0, 7.5, and 15.0 mg/mL concentration of bevacizumab for 24 hours. The 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide and lactate dehydrogenase leakage assays were then performed to assess fibroblast metabolism and viability. The matrix metalloproteinase (MMP), procollagen type I C terminal propeptide (PIP), and laminin immunoassays were performed to examine extracellular matrix production. Changes in cellular morphology were examined by phase-contrast and transmission electron microscopy. Both metabolic activity and viability of primary and recurrent pterygium HTFs were inhibited by bevacizumab in a dose-dependent manner, especially at concentrations greater than 7.5 mg/mL. Both types of HTFs had significant decreases in MMP-1, PIP, and laminin levels. Distinctly, the inhibitory effect of bevacizumab on MMP-1 level related with collagenase in primary pterygium HTFs was significantly higher than that of recurrent pterygium. Significant changes in cellular density and morphology both occurred at bevacizumab concentrations greater than 7.5 mg/mL. Only primary pterygium HTFs had a reduction in cellular density at a bevacizumab concentration of 5.0 mg/mL. Bevacizumab inhibits primary and recurrent pterygium HTFs in a dose-dependent manner, especially at concentrations greater than 7.5 mg/mL. As the primary HTFs produces larger amounts of MMP-1 compared to recurrent HTFs, significant reduction in MMP-1 level in primary pterygium HTFs after exposure to bevacizumab is likely to be related to the faster cellular density changes in primary pterygium HTFs.
Keyword
MeSH Terms
Figure
Reference
-
1. Cameron ME. Histology of pterygium: an electron microscopic study. Br J Ophthalmol. 1983; 67:604–608. PMID: 6882718.
Article3. Turan-Vural E, Torun-Acar B, Kivanc SA, Acar S. The effect of topical 0.05% cyclosporine on recurrence following pterygium surgery. Clin Ophthalmol. 2011; 5:881–885. PMID: 21760716.
Article4. Yalcin Tok O, Burcu Nurozler A, Ergun G, Akbas Kocaoglu F, Duman S. Topical cyclosporine A in the prevention of pterygium recurrence. Ophthalmologica. 2008; 222:391–396. PMID: 18765950.
Article5. Ibáñez M, Eugarrios MF, Calderón DI. Topical cyclosporin A and mitomycin C injection as adjunctive therapy for prevention of primary pterygium recurrence. Ophthalmic Surg Lasers Imaging. 2009; 40:239–244. PMID: 19485286.
Article6. Prabhasawat P, Tesavibul N, Leelapatranura K, Phonjan T. Efficacy of subconjunctival 5-fluorouracil and triamcinolone injection in impending recurrent pterygium. Ophthalmology. 2006; 113:1102–1109. PMID: 16730066.
Article7. Hu Q, Qiao Y, Nie X, Cheng X, Ma Y. Bevacizumab in the treatment of pterygium: a meta-analysis. Cornea. 2014; 33:154–160. PMID: 24326333.8. Ang LP, Chua JL, Tan DT. Current concepts and techniques in pterygium treatment. Curr Opin Ophthalmol. 2007; 18:308–313. PMID: 17568207.
Article9. Paris Fdos S, de Farias CC, Melo GB, Dos Santos MS, Batista JL, Gomes JA. Postoperative subconjunctival corticosteroid injection to prevent pterygium recurrence. Cornea. 2008; 27:406–410. PMID: 18434842.10. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983; 65:55–63. PMID: 6606682.
Article11. Jin J, Guan M, Sima J, Gao G, Zhang M, Liu Z, Fant J, Ma JX. Decreased pigment epithelium-derived factor and increased vascular endothelial growth factor levels in pterygia. Cornea. 2003; 22:473–477. PMID: 12827055.
Article12. Aspiotis M, Tsanou E, Gorezis S, Ioachim E, Skyrlas A, Stefaniotou M, Malamou-Mitsi V. Angiogenesis in pterygium: study of microvessel density, vascular endothelial growth factor, and thrombospondin-1. Eye (Lond). 2007; 21:1095–1101. PMID: 16823458.
Article13. Di Girolamo N, Coroneo MT, Wakefield D. Active matrilysin (MMP-7) in human pterygia: potential role in angiogenesis. Invest Ophthalmol Vis Sci. 2001; 42:1963–1968. PMID: 11481258.14. Lekhanont K, Patarakittam T, Thongphiew P, Suwan-apichon O, Hanutsaha P. Randomized controlled trial of subconjunctival bevacizumab injection in impending recurrent pterygium: a pilot study. Cornea. 2012; 31:155–161. PMID: 22081150.
Article15. Ozgurhan EB, Agca A, Kara N, Yuksel K, Demircan A, Demirok A. Topical application of bevacizumab as an adjunct to recurrent pterygium surgery. Cornea. 2013; 32:835–838. PMID: 23187164.
Article16. van Wijngaarden P, Coster DJ, Williams KA. Inhibitors of ocular neovascularization: promises and potential problems. JAMA. 2005; 293:1509–1513. PMID: 15784876.17. Chalam KV, Agarwal S, Brar VS, Murthy RK, Sharma RK. Evaluation of cytotoxic effects of bevacizumab on human corneal cells. Cornea. 2009; 28:328–333. PMID: 19387236.18. Yoeruek E, Spitzer MS, Tatar O, Aisenbrey S, Bartz-Schmidt KU, Szurman P. Safety profile of bevacizumab on cultured human corneal cells. Cornea. 2007; 26:977–982. PMID: 17721300.
Article19. Miguel NC, Matsuda M, Portes AL, Allodi S, Mendez-Otero R, Puntar T, Sholl-Franco A, Krempel PG, Monteiro ML. In vitro effects of bevacizumab treatment on newborn rat retinal cell proliferation, death, and differentiation. Invest Ophthalmol Vis Sci. 2012; 53:7904–7911. PMID: 23139275.
Article20. O'Neill EC, Qin Q, Van Bergen NJ, Connell PP, Vasudevan S, Coote MA, Trounce IA, Wong TT, Crowston JG. Antifibrotic activity of bevacizumab on human Tenon's fibroblasts in vitro. Invest Ophthalmol Vis Sci. 2010; 51:6524–6532. PMID: 20574016.21. Yong VW, Krekoski CA, Forsyth PA, Bell R, Edwards DR. Matrix metalloproteinases and diseases of the CNS. Trends Neurosci. 1998; 21:75–80. PMID: 9498303.22. Kähäri VM, Saarialho-Kere U. Matrix metalloproteinases and their inhibitors in tumour growth and invasion. Ann Med. 1999; 1:34–45.23. Dushku N, John MK, Schultz GS, Reid TW. Pterygia pathogenesis: corneal invasion by matrix metalloproteinase expressing altered limbal epithelial basal cells. Arch Ophthalmol. 2001; 119:695–706. PMID: 11346397.24. Li DQ, Lee SB, Gunja-Smith Z, Liu Y, Solomon A, Meller D, Tseng SC. Overexpression of collagenase (MMP-1) and stromelysin (MMP-3) by pterygium head fibroblasts. Arch Ophthalmol. 2001; 119:71–80. PMID: 11146729.25. Zeng J, Jiang D, Liu X, Tang L. Expression of matrix metalloproteinase in human pterygia. Yan Ke Xue Bao. 2004; 20:242–245. PMID: 15656370.26. Di Girolamo N, Wakefield D, Coroneo MT. Differential expression of matrix metalloproteinases and their tissue inhibitors at the advancing pterygium head. Invest Ophthalmol Vis Sci. 2000; 41:4142–4149. PMID: 11095607.27. Lee JS, Oum BS, Lee SH. Mitomycin c influence on inhibition of cellular proliferation and subsequent synthesis of type I collagen and laminin in primary and recurrent pterygia. Ophthalmic Res. 2001; 33:140–146. PMID: 11340404.
Article28. Nimmi ME. Fibrillar collagens: their biosynthesis, molecular structure, and mode of assembly. In : Zern MA, Reid LM, editors. Extracellular matrix. New York, NY: Marcel Decker;1993. p. 121–148.29. Risteli L, Risteli J. Noninvasive methods for detection of organ fibrosis. In : Rojkind M, editor. Focus on connective tissue in health and disease. Boca Raton, FL: CRC Press;1990. p. 61–68.30. Timpl R, Rohde H, Robey PG, Rennard SI, Foidart JM, Martin GR. Laminin--a glycoprotein from basement membranes. J Biol Chem. 1979; 254:9933–9937. PMID: 114518.
Article31. Parsian H, Rahimipour A, Nouri M, Somi MH, Qujeq D, Fard MK, Agcheli K. Serum hyaluronic acid and laminin as biomarkers in liver fibrosis. J Gastrointestin Liver Dis. 2010; 19:169–174. PMID: 20593050.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of the Effects Between Bevacizumab and Mitomycin C on the Survival of Fibroblasts
- Effect of Gelrite on the Proliferation of Cultured Human Tenon's Capsule Fibroblasts
- Clinical Results After Application of Bevacizumab in Recurrent Pterygium
- Effects of Subconjunctival Bevacizumab Injection after Primary Pterygium Surgery
- The Effect of Subconjunctival Bevacizumab Injection after Primary Pterygium Surgery