Korean J Physiol Pharmacol.
2015 Mar;19(2):177-181. 10.4196/kjpp.2015.19.2.177.
Low Non-NMDA Receptor Current Density as Possible Protection Mechanism from Neurotoxicity of Circulating Glutamate on Subfornical Organ Neurons in Rats
- Affiliations
-
- 1Department of Veterinary Pharmacology, College of Veterinary Medicine, Seoul National University, Seoul 151-742, Korea. pdryu@snu.ac.kr
- 2Department of Oral Physiology, School of Dentistry and Institute of Oral Bioscience, Chonbuk National University, Jeonju 561-756, Korea.
- KMID: 2285570
- DOI: http://doi.org/10.4196/kjpp.2015.19.2.177
Abstract
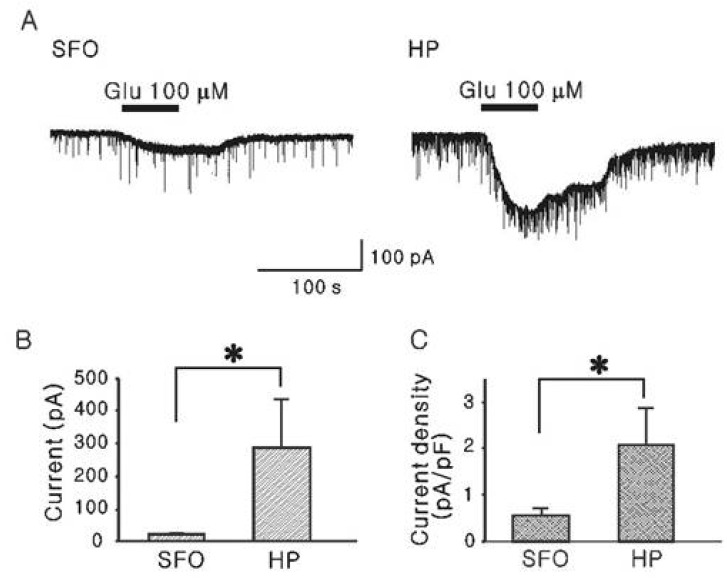
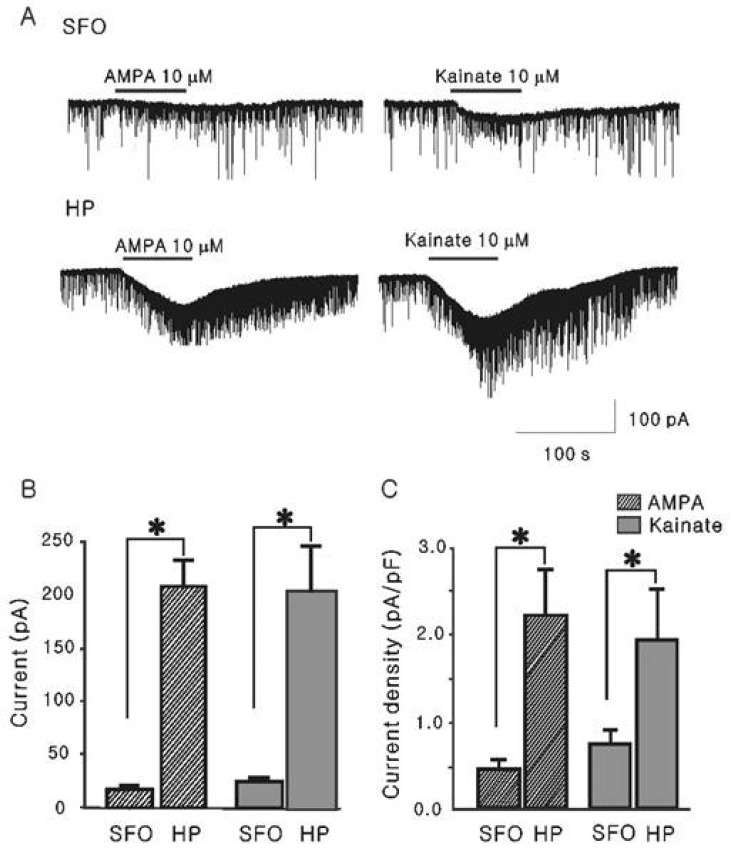
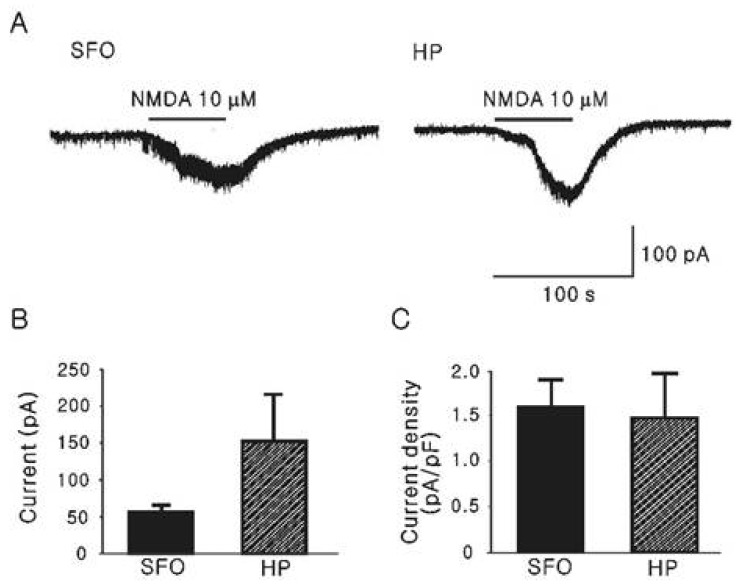
- The subfornical organ (SFO) is one of circumventricular organs characterized by the lack of a normal blood brain barrier. The SFO neurons are exposed to circulating glutamate (60~100 microM), which may cause excitotoxicity in the central nervous system. However, it remains unclear how SFO neurons are protected from excitotoxicity caused by circulating glutamate. In this study, we compared the glutamate-induced whole cell currents in SFO neurons to those in hippocampal CA1 neurons using the patch clamp technique in brain slice. Glutamate (100 microM) induced an inward current in both SFO and hippocampal CA1 neurons. The density of glutamate-induced current in SFO neurons was significantly smaller than that in hippocampal CA1 neurons (0.55 vs. 2.07 pA/pF, p<0.05). To further identify the subtype of the glutamate receptors involved, the whole cell currents induced by selective agonists were then compared. The current densities induced by AMPA (0.45 pA/pF) and kainate (0.83 pA/pF), non-NMDA glutamate receptor agonists in SFO neurons were also smaller than those in hippocampal CA1 neurons (2.44 pA/pF for AMPA, p<0.05; 2.34 pA/pF for kainate, p< 0.05). However, the current density by NMDA in SFO neurons was not significantly different from that of hippocampal CA1 neurons (1.58 vs. 1.47 pA/pF, p>0.05). These results demonstrate that glutamate-mediated action through non-NMDA glutamate receptors in SFO neurons is smaller than that of hippocampal CA1 neurons, suggesting a possible protection mechanism from excitotoxicity by circulating glutamate in SFO neurons.
MeSH Terms
-
alpha-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic Acid
Animals
Blood-Brain Barrier
Brain
Central Nervous System
Glutamic Acid*
Hippocampus
Kainic Acid
N-Methylaspartate
Neurons*
Rats*
Receptors, Glutamate
Subfornical Organ*
Glutamic Acid
Kainic Acid
N-Methylaspartate
Receptors, Glutamate
alpha-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic Acid
Figure
Cited by 1 articles
-
The neuroprotective mechanism of ampicillin in a mouse model of transient forebrain ischemia
Kyung-Eon Lee, Kyung-Ok Cho, Yun-Sik Choi, Seong Yun Kim
Korean J Physiol Pharmacol. 2016;20(2):185-192. doi: 10.4196/kjpp.2016.20.2.185.
Reference
-
1. Dellmann HD. Structure of the subfornical organ: a review. Microsc Res Tech. 1998; 41:85–97. PMID: 9579597.
Article2. Johnson AK, Gross PM. Sensory circumventricular organs and brain homeostatic pathways. FASEB J. 1993; 7:678–686. PMID: 8500693.
Article3. Ferguson AV, Bains JS. Actions of angiotensin in the subfornical organ and area postrema: implications for long term control of autonomic output. Clin Exp Pharmacol Physiol. 1997; 24:96–101. PMID: 9043813.
Article4. McKinley MJ, Allen AM, Burns P, Colvill LM, Oldfield BJ. Interaction of circulating hormones with the brain: the roles of the subfornical organ and the organum vasculosum of the lamina terminalis. Clin Exp Pharmacol Physiol Suppl. 1998; 25:S61–S67. PMID: 9809195.
Article5. Smith PM, Chambers AP, Price CJ, Ho W, Hopf C, Sharkey KA, Ferguson AV. The subfornical organ: a central nervous system site for actions of circulating leptin. Am J Physiol Regul Integr Comp Physiol. 2009; 296:R512–R520. PMID: 19020290.
Article6. Price MT, Olney JW, Lowry OH, Buchsbaum S. Uptake of exogenous glutamate and aspartate by circumventricular organs but not other regions of brain. J Neurochem. 1981; 36:1774–1780. PMID: 6113269.
Article7. Fontana L, Moreira E, Torres MI, Fernández MI, Ríos A, Sánchez de Medina F, Gil A. Serum amino acid changes in rats with thioacetamide-induced liver cirrhosis. Toxicology. 1996; 106:197–206. PMID: 8571392.
Article8. Hawkins RA, DeJoseph MR, Hawkins PA. Regional brain glutamate transport in rats at normal and raised concentrations of circulating glutamate. Cell Tissue Res. 1995; 281:207–214. PMID: 7648616.
Article9. Gutman MB, Ciriello J, Mogenson GJ. Effect of paraventricular nucleus lesions on cardiovascular responses elicited by stimulation of the subfornical organ in the rat. Can J Physiol Pharmacol. 1985; 63:816–824. PMID: 2864128.
Article10. Osaka T, Yamashita H, Koizumi K. Inhibitory inputs to the subfornical organ from the AV3V: involvement of GABA. Brain Res Bull. 1992; 29:581–587. PMID: 1422855.
Article11. Schmid HA. Effect of glutamate and angiotensin II on whole cell currents and release of nitric oxide in the rat subfornical organ. Amino Acids. 1998; 14:113–119. PMID: 9871450.
Article12. Weindl A, Bufler J, Winkler B, Arzberger T, Hatt H. Neurotransmitters and receptors in the subfornical organ. Immunohistochemical and electrophysiological evidence. Prog Brain Res. 1992; 91:261–269. PMID: 1329146.13. Xu SH, Inenaga K, Honda E, Yamashita H. Glutamatergic synaptic inputs activate neurons in the subfornical organ through non-NMDA receptors. J Auton Nerv Syst. 2000; 78:177–180. PMID: 10789698.
Article14. Lee HS, Chong W, Han SK, Lee MH, Ryu PD. Activation of metabotropic glutamate receptors inhibits GABAergic transmission in the rat subfornical organ. Neuroscience. 2001; 102:401–411. PMID: 11166126.
Article15. Corrêa FM, Saavedra JM. Chemical lesion of the circumventricular organs with monosodium glutamate reduces the blood pressure of spontaneously hypertensive but not of one kidney-one clip hypertensive rats. Braz J Med Biol Res. 1992; 25:515–519. PMID: 1342228.16. Choi DW, Rothman SM. The role of glutamate neurotoxicity in hypoxic-ischemic neuronal death. Annu Rev Neurosci. 1990; 13:171–182. PMID: 1970230.
Article17. Ozawa S, Kamiya H, Tsuzuki K. Glutamate receptors in the mammalian central nervous system. Prog Neurobiol. 1998; 54:581–618. PMID: 9550192.
Article18. Harry GJ, Lefebvre d'Hellencourt C. Dentate gyrus: alterations that occur with hippocampal injury. Neurotoxicology. 2003; 24:343–356. PMID: 12782100.
Article19. Schmidt-Kastner R, Freund TF. Selective vulnerability of the hippocampus in brain ischemia. Neuroscience. 1991; 40:599–636. PMID: 1676492.
Article20. Inenaga K, Nagatomo T, Honda E, Ueta Y, Yamashita H. GABAergic inhibitory inputs to subfornical organ neurons in rat slice preparations. Brain Res. 1995; 705:85–90. PMID: 8821737.
Article21. Ferguson AV, Li Z. Whole cell patch recordings from forebrain slices demonstrate angiotensin II inhibits potassium currents in subfornical organ neurons. Regul Pept. 1996; 66:55–58. PMID: 8899894.
Article22. Bekkers JM, Stevens CF. NMDA receptors at excitatory synapses in the hippocampus: test of a theory of magnesium block. Neurosci Lett. 1993; 156:73–77. PMID: 8414193.
Article23. Takasaki Y. Studies on brain lesions after administration of monosodium L-glutamate to mice. II. Absence of brain damage following administration of monosodium L-glutamate in the diet. Toxicology. 1978; 9:307–318. PMID: 663940.
Article24. Simonian NA, Getz RL, Leveque JC, Konradi C, Coyle JT. Kainic acid induces apoptosis in neurons. Neuroscience. 1996; 75:1047–1055. PMID: 8938740.
Article25. Portera-Cailliau C, Price DL, Martin LJ. Non-NMDA and NMDA receptor-mediated excitotoxic neuronal deaths in adult brain are morphologically distinct: further evidence for an apoptosis-necrosis continuum. J Comp Neurol. 1997; 378:88–104. PMID: 9120056.
Article26. Michaelis EK. Molecular biology of glutamate receptors in the central nervous system and their role in excitotoxicity, oxidative stress and aging. Prog Neurobiol. 1998; 54:369–415. PMID: 9522394.
Article27. Osaka T, Yamashita H, Koizumi K. Inhibition of paraventricular neurons by subfornical organ and AV3V in cats. Am J Physiol. 1988; 255:R961–R967. PMID: 3202228.
Article28. Osaka T, Yamashita H, Koizumi K. Inhibitory inputs to the subfornical organ from the AV3V: involvement of GABA. Brain Res Bull. 1992; 29:581–587. PMID: 1422855.
Article29. Wright JW, Roberts KA, Stubley LA, Hanesworth JM, Harding JW. Hypothalamic angiotensin release in response to AII or glutamic acid stimulation of the SFO in rats. Brain Res Bull. 1993; 31:649–654. PMID: 8100178.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Anethesia and Glutamate Receptors
- Effect of Brain-derived Neurotrophic Factor on Excitatory Synaptic Transmission in Hippocampal Neurons
- Mechanism of Glutamate-induced[Ca2+]i Increase in Substantia Gelatinosa Neurons of Juvenile Rats
- The Study on the Role of NMDA Receptors in Rat Visual Cortex
- The Effects of N-Methyl_D-Aspartic Acid, and Antagonism by Kynurenic Acid on Neurons in the Cathish Retina