Korean J Physiol Pharmacol.
2014 Jun;18(3):241-247. 10.4196/kjpp.2014.18.3.241.
Effects of C18 Fatty Acids on Intracellular Ca2+ Mobilization and Histamine Release in RBL-2H3 Cells
- Affiliations
-
- 1College of Pharmacy, Chung-Ang University, Seoul 156-756, Korea. simss@cau.ac.kr
- KMID: 2285516
- DOI: http://doi.org/10.4196/kjpp.2014.18.3.241
Abstract
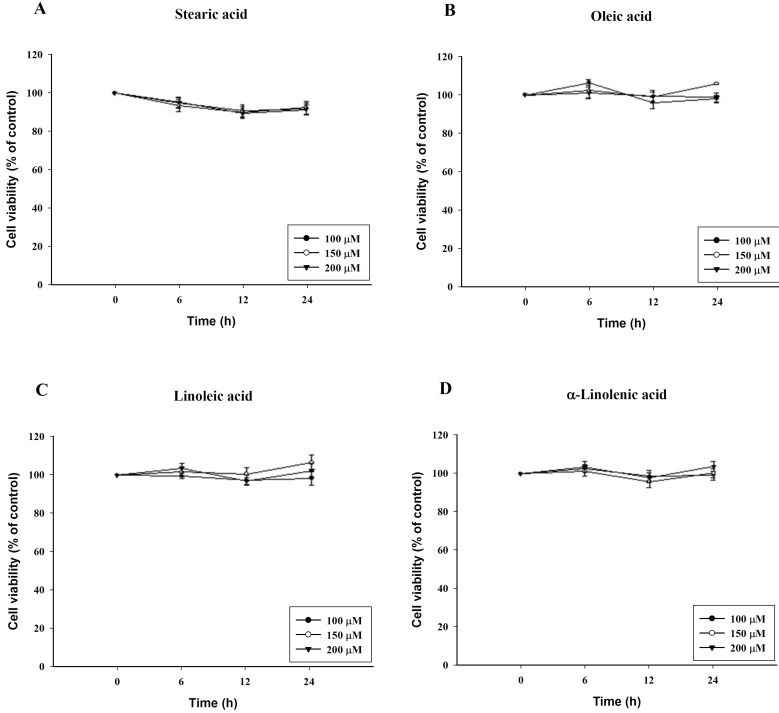
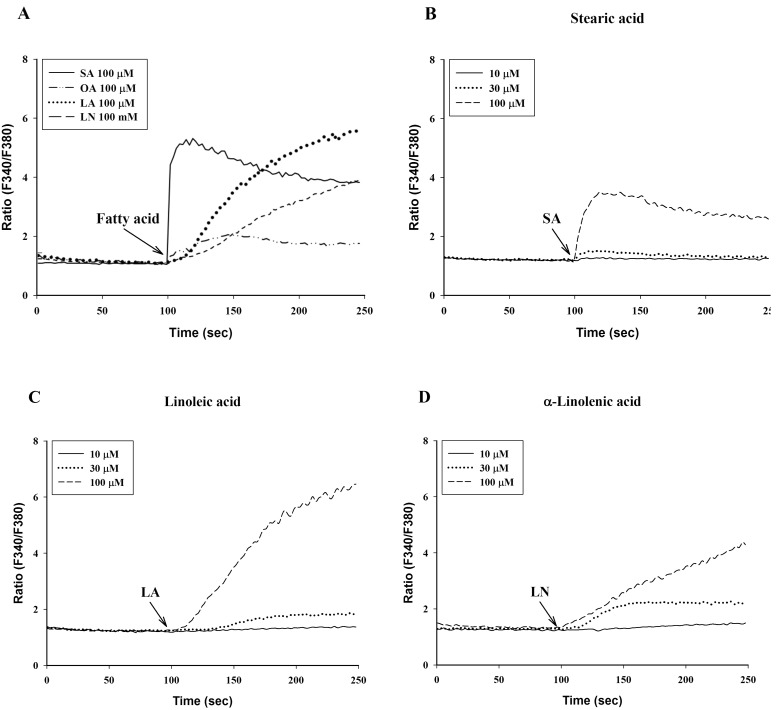
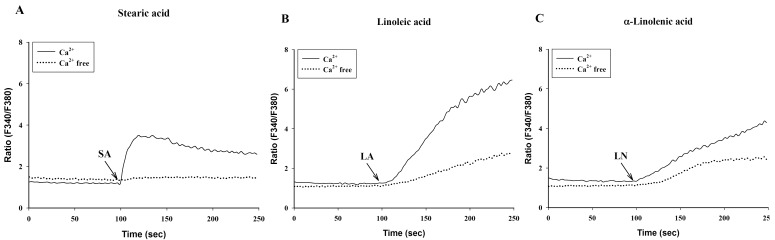
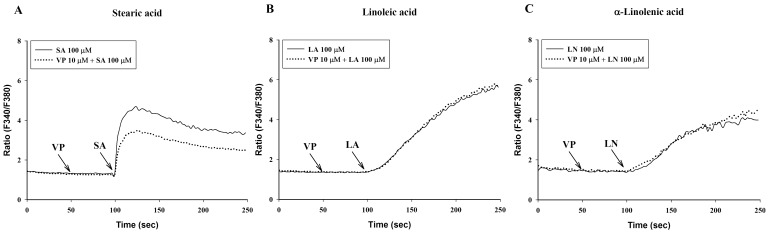
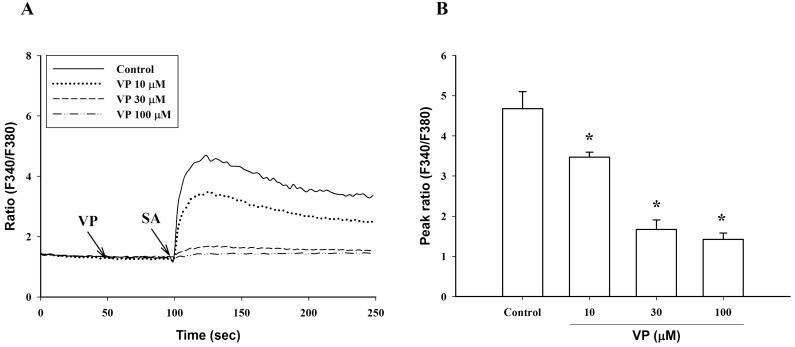
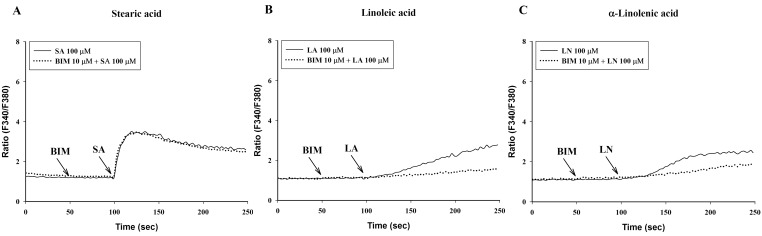
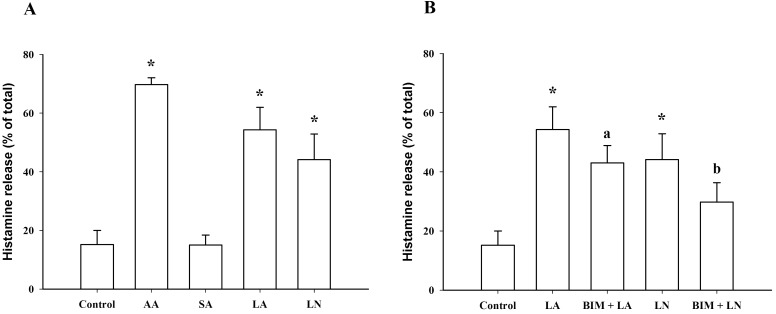
- To investigate the underlying mechanisms of C18 fatty acids (stearic acid, oleic acid, linoleic acid and alpha-linolenic acid) on mast cells, we measured the effect of C18 fatty acids on intracellular Ca2+ mobilization and histamine release in RBL-2H3 mast cells. Stearic acid rapidly increased initial peak of intracellular Ca2+ mobilization, whereas linoleic acid and alpha-linolenic acid gradually increased this mobilization. In the absence of extracellular Ca2+, stearic acid (100 microM) did not cause any increase of intracellular Ca2+ mobilization. Both linoleic acid and alpha-linolenic acid increased intracellular Ca2+ mobilization, but the increase was smaller than that in the presence of extracellular Ca2+. These results suggest that C18 fatty acid-induced intracellular Ca2+ mobilization is mainly dependent on extracellular Ca2+ influx. Verapamil dose-dependently inhibited stearic acid-induced intracellular Ca2+ mobilization, but did not affect both linoleic acid and alpha-linolenic acid-induced intracellular Ca2+ mobilization. These data suggest that the underlying mechanism of stearic acid, linoleic acid and alpha-linolenic acid on intracellular Ca2+ mobilization may differ. Linoleic acid and alpha-linolenic acid significantly increased histamine release. Linoleic acid (C18:2: omega-6)-induced intracellular Ca2+ mobilization and histamine release were more prominent than alpha-linolenic acid (C18:3: omega-3). These data support the view that the intake of more alpha-linolenic acid than linoleic acid is useful in preventing inflammation.
MeSH Terms
Figure
Reference
-
1. Marshall JS. Mast-cell responses to pathogens. Nat Rev Immunol. 2004; 4:787–799. PMID: 15459670.
Article2. Nakano N, Nakao A, Uchida T, Shirasaka N, Yoshizumi H, Okumura K, Tsuboi R, Ogawa H. Effects of arachidonic acid analogs on FcepsilonRI-mediated activation of mast cells. Biochim Biophys Acta. 2005; 1738:19–28. PMID: 16403671.3. Lee JH, Lee JY, Kang HS, Jeong CH, Moon H, Whang WK, Kim CJ, Sim SS. The effect of acteoside on histamine release and arachidonic acid release in RBL-2H3 mast cells. Arch Pharm Res. 2006; 29:508–513. PMID: 16833020.
Article4. Calder PC. Polyunsaturated fatty acids and inflammation. Prostaglandins Leukot Essent Fatty Acids. 2006; 75:197–202. PMID: 16828270.
Article5. Babcock TA, Novak T, Ong E, Jho DH, Helton WS, Espat NJ. Modulation of lipopolysaccharide-stimulated macrophage tumor necrosis factor-alpha production by omega-3 fatty acid is associated with differential cyclooxygenase-2 protein expression and is independent of interleukin-10. J Surg Res. 2002; 107:135–139. PMID: 12384076.6. Woerdenbag HJ, Merfort I, Passreiter CM, Schmidt TJ, Willuhn G, van Uden W, Pras N, Kampinga HH, Konings AW. Cytotoxicity of flavonoids and sesquiterpene lactones from Arnica species against the GLC4 and the COLO 320 cell lines. Planta Med. 1994; 60:434–437. PMID: 7997472.7. Lee MJ, Song HJ, Jeong JY, Park SY, Sohn UD. Anti-Oxidative and Anti-Inflammatory Effects of QGC in Cultured Feline Esophageal Epithelial Cells. Korean J Physiol Pharmacol. 2013; 17:81–87. PMID: 23440684.
Article8. Tseng PH, Lin HP, Hu H, Wang C, Zhu MX, Chen CS. The canonical transient receptor potential 6 channel as a putative phosphatidylinositol 3,4,5-trisphosphate-sensitive calcium entry system. Biochemistry. 2004; 43:11701–11708. PMID: 15362854.
Article9. Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985; 260:3440–3450. PMID: 3838314.10. Hwang YH, Song HS, Kim HR, Ko MS, Jeong JM, Kim YH, Ryu JS, Sohn UD, Gimm YM, Myung SH, Sim SS. Intracellular Ca Mobilization and Beta-hexosaminidase Release Are Not Influenced by 60 Hz-electromagnetic Fields (EMF) in RBL 2H3 Cells. Korean J Physiol Pharmacol. 2011; 15:313–317. PMID: 22128265.11. Bae YS, Lee TG, Park JC, Hur JH, Kim Y, Heo K, Kwak JY, Suh PG, Ryu SH. Identification of a compound that directly stimulates phospholipase C activity. Mol Pharmacol. 2003; 63:1043–1050. PMID: 12695532.
Article12. Håkanson R, Rönnberg AL, Sjölund K. Fluorometric determination of histamine with OPT: optimum reaction conditions and tests of identity. Anal Biochem. 1972; 47:356–370. PMID: 5036454.
Article13. Masi LN, Portioli-Sanches EP, Lima-Salgado TM, Curi R. Toxicity of fatty acids on ECV-304 endothelial cells. Toxicol In Vitro. 2011; 25:2140–2146. PMID: 21723937.
Article14. Teshima R, Amano F, Nakamura R, Tanaka Y, Sawada J. Effects of polyunsaturated fatty acids on calcium response and degranulation from RBL-2H3 cells. Int Immunopharmacol. 2007; 7:205–210. PMID: 17178388.
Article15. Baliño P, Pastor R, Aragon CM. Participation of L-type calcium channels in ethanol-induced behavioral stimulation and motor incoordination: effects of diltiazem and verapamil. Behav Brain Res. 2010; 209:196–204. PMID: 20122967.
Article16. Ichimura A, Hirasawa A, Hara T, Tsujimoto G. Free fatty acid receptors act as nutrient sensors to regulate energy homeostasis. Prostaglandins Other Lipid Mediat. 2009; 89:82–88. PMID: 19460454.
Article17. Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, Li P, Lu WJ, Watkins SM, Olefsky JM. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010; 142:687–698. PMID: 20813258.
Article18. Griffin BA. How relevant is the ratio of dietary n-6 to n-3 polyunsaturated fatty acids to cardiovascular disease risk? Evidence from the OPTILIP study. Curr Opin Lipidol. 2008; 19:57–62. PMID: 18196988.
Article19. Lands WE. Biochemistry and physiology of n-3 fatty acids. FASEB J. 1992; 6:2530–2536. PMID: 1592205.
Article20. Okuyama H. High n-6 to n-3 ratio of dietary fatty acids rather than serum cholesterol as a major risk factor for coronary heart disease. Eur J Lipid Sci Technol. 2001; 103:418–422.
Article21. Willett WC. The role of dietary n-6 fatty acids in the prevention of cardiovascular disease. J Cardiovasc Med (Hagerstown). 2007; 8(Suppl 1):S42–S45. PMID: 17876199.
Article22. Mozaffarian D, Ascherio A, Hu FB, Stampfer MJ, Willett WC, Siscovick DS, Rimm EB. Interplay between different polyunsaturated fatty acids and risk of coronary heart disease in men. Circulation. 2005; 111:157–164. PMID: 15630029.
Article23. Hooper L, Thompson RL, Harrison RA, Summerbell CD, Ness AR, Moore HJ, Worthington HV, Durrington PN, Higgins JP, Capps NE, Riemersma RA, Ebrahim SB, Davey Smith G. Risks and benefits of omega 3 fats for mortality, cardiovascular disease, and cancer: systematic review. BMJ. 2006; 332:752–760. PMID: 16565093.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Intracellular Ca2+ Mobilization and Beta-hexosaminidase Release Are Not Influenced by 60 Hz-electromagnetic Fields (EMF) in RBL 2H3 Cells
- Imidacloprid inhibits IgE-mediated RBL-2H3 cell degranulation and passive cutaneous anaphylaxis
- A Synthetic Chenodeoxycholic Acid Derivative, HS-1200-induced Apoptosis of RBL-2H3 Cells
- Fluoxetine-induced Changes on Activity of Tryptophan Hydroxylase at RBL-2H3 Cells
- Inhibition of Interleukin-4 and β-Hexosaminidase Release in RBL-2H3 Cells by Compounds Isolated from Lobelia chinensis