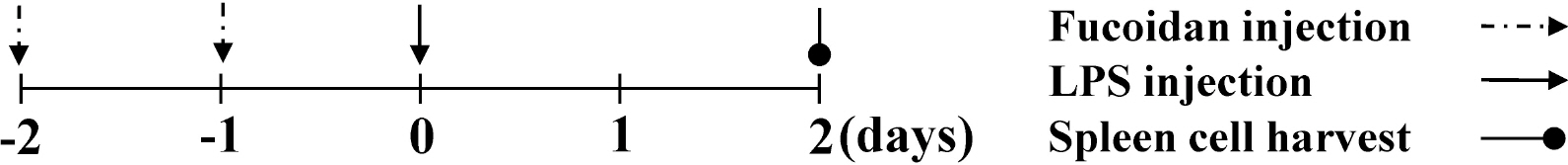Korean J Physiol Pharmacol.
2011 Apr;15(2):89-94. 10.4196/kjpp.2011.15.2.89.
Fucoidan Enhances the Survival and Sustains the Number of Splenic Dendritic Cells in Mouse Endotoxemia
- Affiliations
-
- 1Laboratory of Veterinary Pharmacology, College of Veterinary Medicine, Jeju 690-756, Korea. jooh@jejunu.ac.kr
- 2Veterinary Medical Research Institute, Jeju National University, Jeju 690-756, Korea.
- KMID: 2285412
- DOI: http://doi.org/10.4196/kjpp.2011.15.2.89
Abstract
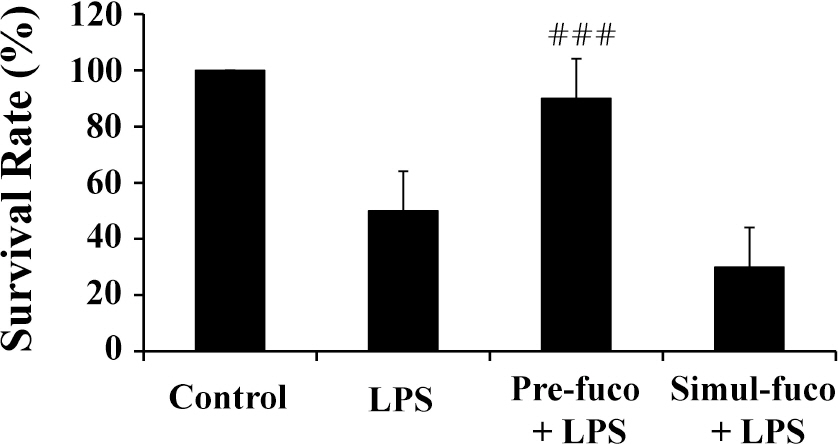
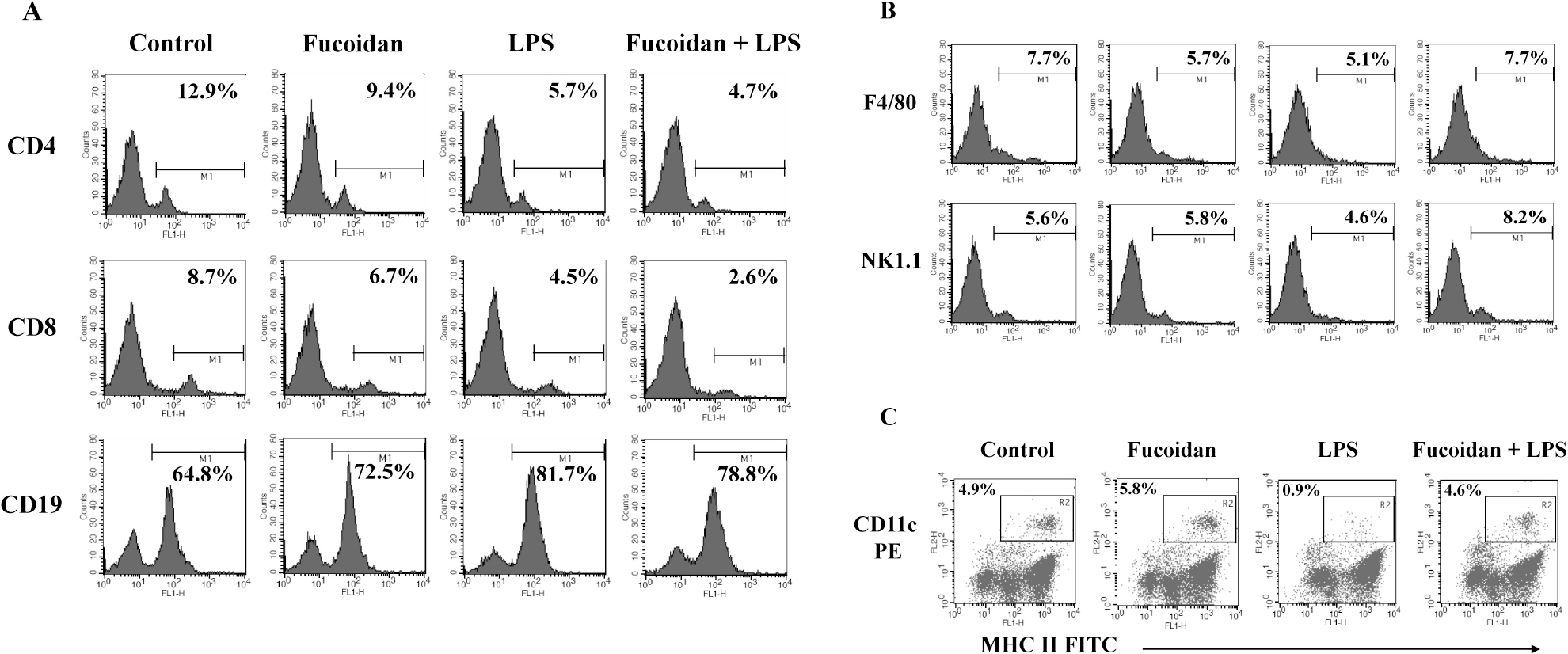
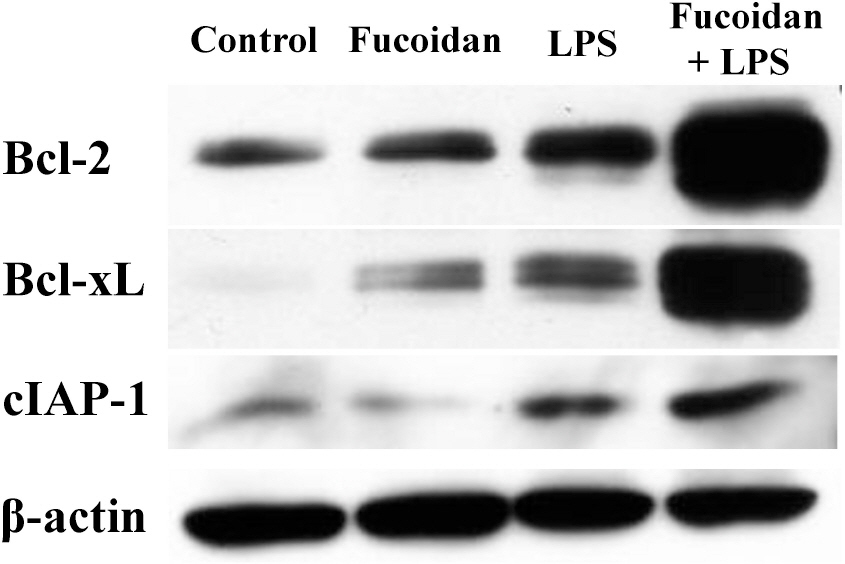
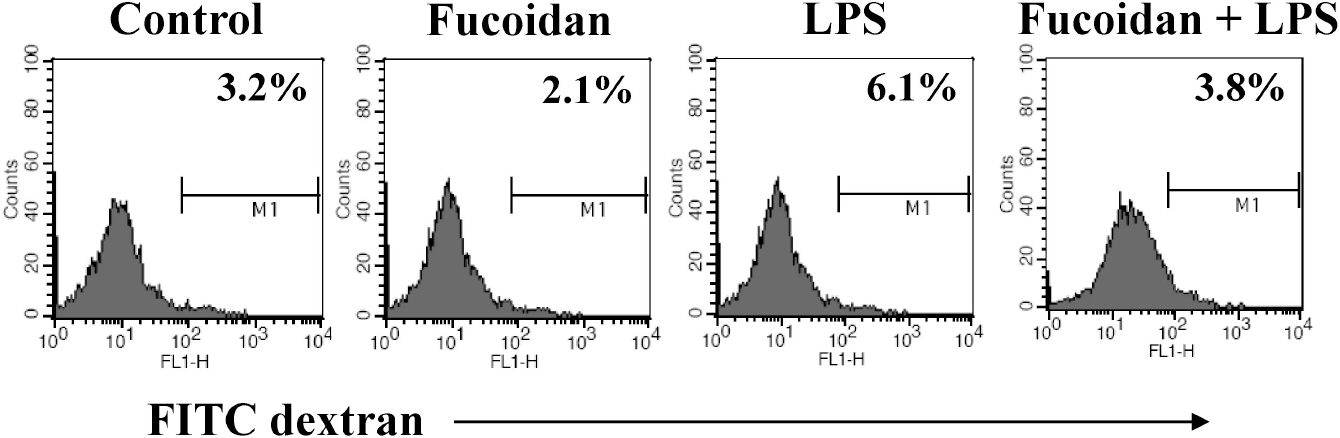
- Fucoidan is a sulfated polysaccharide derived from brown algae that has been reported to perform multiple biological activities, including immunostimulation. In this study, we investigated whether fucoidan has beneficial effects on endotoxemia induced by LPS, a septic model in mice. The focus of this study was on survival rates and spleen function of the mice upon treatment. We found that fucoidan had prophylactic effects on the survival rate of mice with endotoxemia. Flow cytometric analysis using antibodies for subset-specific markers revealed that fucoidan profoundly reversed the depleted population of dendritic cells in mice with endotoxemia. According to Western blot analysis, the spleen cells of LPS/fucoidan-treated mice showed a higher expression of anti-apoptotic molecules compared to those of LPS-treated mice. Also, fucoidan-treated spleen cells were more responsive to mitogens. Taken together, these results demonstrate that fucoidan pre-treatment has beneficial effects on the survival rate and function of the spleen in mice with endotoxemia. This study may broaden the use of fucoidan in clinical fields, especially endotoxemia.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Evaluation of adjuvant effects of fucoidan for improving vaccine efficacy
Su-Yeon Kim, Hong-Gu Joo
J Vet Sci. 2015;16(2):145-150. doi: 10.4142/jvs.2015.16.2.145.Fucoidan Promotes the Reconstruction of Skin Equivalents
Yu Seok Song, Hailan Li, Marie Carmel Balcos, Hye-Young Yun, Kwang Jin Baek, Nyoun Soo Kwon, Hye-Ryung Choi, Kyoung-Chan Park, Dong-Seok Kim
Korean J Physiol Pharmacol. 2014;18(4):327-331. doi: 10.4196/kjpp.2014.18.4.327.
Reference
-
References
1. Byon YY, Kim MH, Yoo ES, Hwang KK, Jee Y, Shin T, Joo HG. Radioprotective effects of fucoidan on bone marrow cells: improvement of the cell survival and immunoreactivity. J Vet Sci. 2008; 9:359–365.
Article2. Frenette PS, Weiss L. Sulfated glycans induce rapid hematopoietic progenitor cell mobilization: evidence for selectin-dependent and independent mechanisms. Blood. 2000; 96:2460–2468.
Article3. Oomizu S, Yanase Y, Suzuki H, Kameyoshi Y, Hide M. Fucoidan prevents C epsilon germline transcription and NFkappaB p52 translocation for IgE production in B cells. Biochem Biophys Res Commun. 2006; 350:501–507.4. Kim MH, Joo HG. Immunostimulatory effects of fucoidan on bone marrow-derived dendritic cells. Immunol Lett. 2008; 115:138–143.
Article5. Maruyama H, Tamauchi H, Iizuka M, Nakano T. The role of NK cells in antitumor activity of dietary fucoidan from Undaria pinnatifida sporophylls (Mekabu). Planta Med. 2006; 72:1415–1417.
Article6. Zen K, Liu Y, Cairo D, Parkos CA. CD11b/CD18-dependent interactions of neutrophils with intestinal epithelium are mediated by fucosylated proteoglycans. J Immunol. 2002; 169:5270–5278.
Article7. Cohen J. The immunopathogenesis of sepsis. Nature. 2002; 420:885–891.
Article8. Lyn-Kew K, Standiford TJ. Immunosuppression in sepsis. Curr Pharm Des. 2008; 14:1870–1881.
Article9. Tinsley KW, Grayson MH, Swanson PE, Drewry AM, Chang KC, Karl IE, Hotchkiss RS. Sepsis induces apoptosis and profound depletion of splenic interdigitating and follicular dendritic cells. J Immunol. 2003; 171:909–914.
Article10. Hotchkiss RS, Tinsley KW, Swanson PE, Grayson MH, Osborne DF, Wagner TH, Cobb JP, Coopersmith C, Karl IE. Depletion of dendritic cells, but not macrophages, in patients with sepsis. J Immunol. 2002; 168:2493–2500.
Article11. Joo HG, Goedegebuure PS, Sadanaga N, Nagoshi M, von Bernstorff W, Eberlein TJ. Expression and function of galectin-3, a beta-galactoside-binding protein in activated T lymphocytes. J Leukoc Biol. 2001; 69:555–564.12. Kim MH, Byon YY, Ko EJ, Song JY, Yun YS, Shin T, Joo HG. Immunomodulatory activity of ginsan, a polysaccharide of Panax ginseng, on dendritic cells. Korean J Physiol Pharmacol. 2009; 13:169–173.13. Ley K, Linnemann G, Meinen M, Stoolman LM, Gaehtgens P. Fucoidin, but not yeast polyphosphomannan PPME, inhibits leukocyte rolling in venules of the rat mesentery. Blood. 1993; 81:177–185.
Article14. Granert C, Raud J, Xie X, Lindquist L, Lindbom L. Inhibition of leukocyte rolling with polysaccharide fucoidin prevents pleocytosis in experimental meningitis in the rabbit. J Clin Invest. 1994; 93:929–936.
Article15. Thorlacius H, Vollmar B, Seyfert UT, Vestweber D, Menger MD. The polysaccharide fucoidan inhibits microvascular thrombus formation independently from P- and L-selectin function in vivo. Eur J Clin Invest. 2000; 30:804–810.16. Hofstad T, Skaug N, Sveen K. Stimulation of B lymphocytes by lipopolysaccharides from anaerobic bacteria. Clin Infect Dis. 1993; 16 Suppl. 4:S200–202.
Article17. Zhang J, Qu JM, He LX. IL-12 suppression, enhanced endocytosis and up-regulation of MHC-II and CD80 in dendritic cells during experimental endotoxin tolerance. Acta Pharmacol Sin. 2009; 30:582–588.
Article18. Wheeler DS, Lahni PM, Denenberg AG, Poynter SE, Wong HR, Cook JA, Zingarelli B. Induction of endotoxin tolerance enhances bacterial clearance and survival in murine polymicrobial sepsis. Shock. 2008; 30:267–273.
Article19. Nishikawa K, Arai H, Inoue K. Scavenger receptor-mediated uptake and metabolism of lipid vesicles containing acidic phospholipids by mouse peritoneal macrophages. J Biol Chem. 1990; 265:5226–5231.
Article20. Bochkov VN, Tkachuk VA, Philippova MP, Stambolsky DV, Bühler FR, Resink TJ. Ligand selectivity of 105 kDa and 130 kDa lipoprotein-binding proteins in vascular-smooth-muscle-cell membranes is unique. Biochem J. 1996; 317:297–304.
Article21. Pierini LM. Uptake of serum-opsonized Francisella tularensis by macrophages can be mediated by class A scavenger receptors. Cell Microbiol. 2006; 8:1361–1370.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pro-inflammatory Cytokine Expression of Spleen Dendritic Cells in Mouse Toxoplasmosis
- Expression of TNF-alpha and IL-1beta in Splenic Dendritic Cells and Their Serum Levels in Mouse Sparganosis
- Biological effects of fucoidan isolated from Fucus vesiculosus on thrombosis and vascular cells
- Radioprotective effects of fucoidan on bone marrow cells: improvement of the cell survival and immunoreactivity
- Immunohistochemical Characterization on the Effect of Immunomodulating Factor (IMF) from Actinobacillus actinomycetemcomitans on Dendritic Cells, Lymphocytes and Macrophages in the Mouse Spleen