J Adv Prosthodont.
2013 May;5(2):84-91. 10.4047/jap.2013.5.2.84.
Physical stability of arginine-glycine-aspartic acid peptide coated on anodized implants after installation
- Affiliations
-
- 1Department of Prosthodontics, School of Dentistry, Dental Hospital, Dental Research Institute, Pusan National University, Yangsan, Republic of Korea.
- 2Department of Prosthodontics, Institute for Clinical Dental Research, Korea University Guro Hospital, Korea University, Seoul, Republic of Korea.
- 3Department of Dental Biomaterials Science and Dental Research Institute, School of Dentistry, Seoul National University, Seoul, Republic of Korea. 937331@paran.com
- 4Department of Prosthodontics, Institute for Clinical Dental Research, Korea University Anam Hospital, Korea University, Seoul, Republic of Korea. koprosth@unitel.co.kr
- KMID: 2284745
- DOI: http://doi.org/10.4047/jap.2013.5.2.84
Abstract
- PURPOSE
The aim of this study was to evaluate the stability of arginine-glycine-aspartic acid (RGD) peptide coatings on implants by measuring the amount of peptide remaining after installation.
MATERIALS AND METHODS
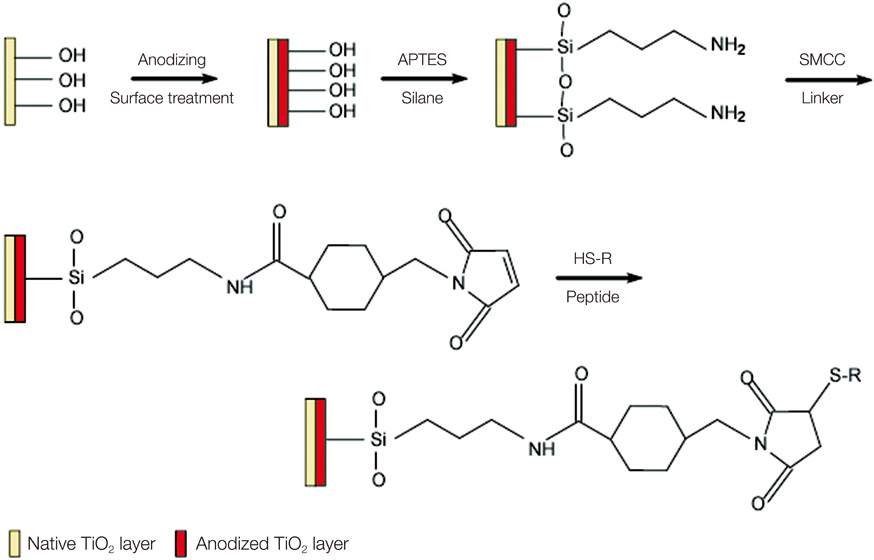
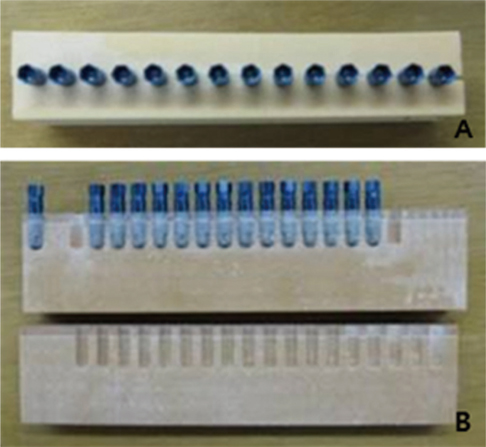
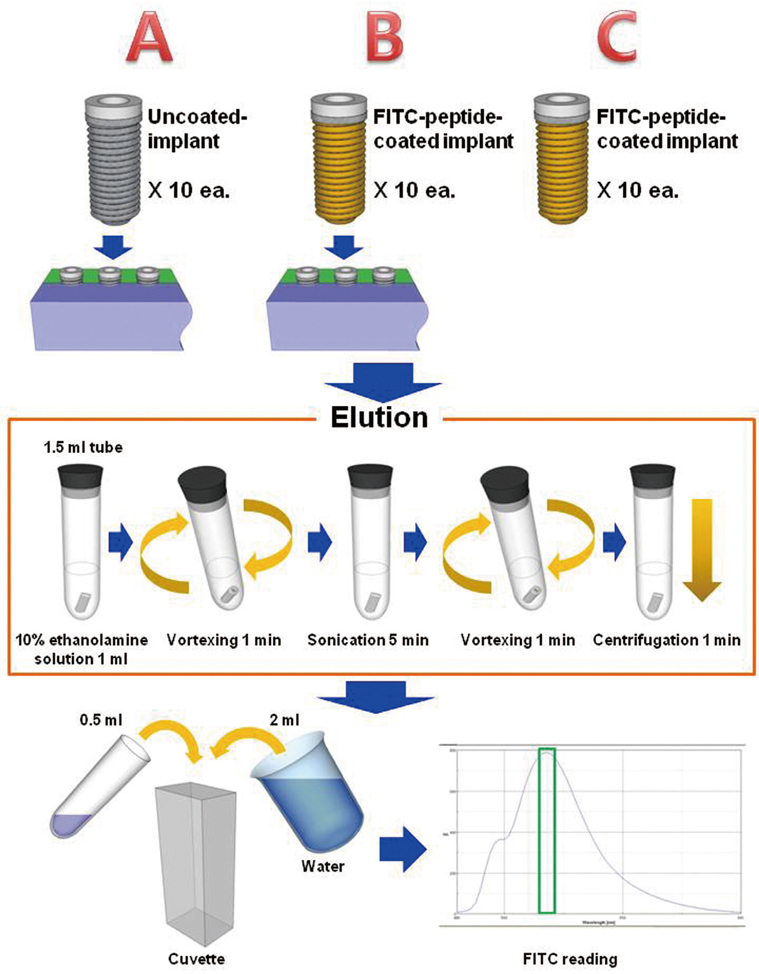
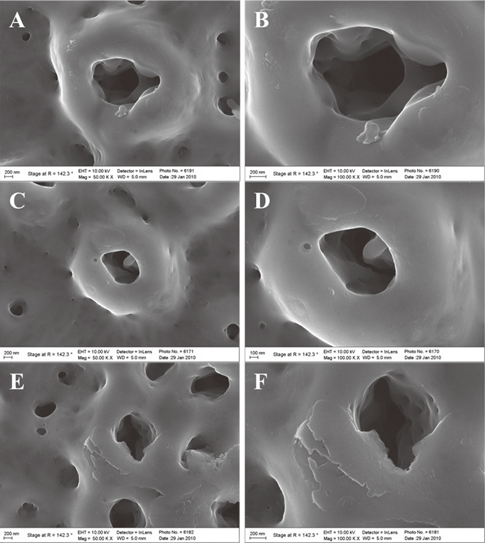
Fluorescent isothiocyanate (FITC)-fixed RGD peptide was coated onto anodized titanium implants (width 4 mm, length 10 mm) using a physical adsorption method (P) or a chemical grafting method (C). Solid Rigid Polyurethane Foam (SRPF) was classified as either hard bone (H) or soft bone (S) according to its density. Two pieces of artificial bone were fixed in a customized jig, and coated implants were installed at the center of the boundary between two pieces of artificial bone. The test groups were classified as: P-H, P-S, C-H, or C-S. After each installation, implants were removed from the SRPF, and the residual amounts and rates of RGD peptide in implants were measured by fluorescence spectrometry. The Kruskal-Wallis test was used for the statistical analysis (alpha=0.05).
RESULTS
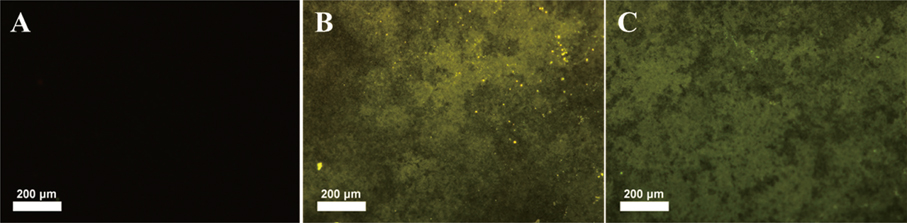
Peptide-coating was identified by fluorescence microscopy and XPS. Total coating amount was higher for physical adsorption than chemical grafting. The residual rate of peptide was significantly larger in the P-S group than in the other three groups (P<.05).
CONCLUSION
The result of this study suggests that coating doses depend on coating method. Residual amounts of RGD peptide were greater for the physical adsorption method than the chemical grafting method.
MeSH Terms
Figure
Cited by 1 articles
-
Effect of immobilization of the recombinant human bone morphogenetic protein 2 (rhBMP-2) on anodized implants coated with heparin for improving alveolar ridge augmentation in beagle dogs: Radiographic observations
So-Hyoun Lee, Jae-Young Jo, Mi-Jung Yun, Young-Chan Jeon, Jung-Bo Huh, Chang-Mo Jeong
J Korean Acad Prosthodont. 2013;51(4):307-314. doi: 10.4047/jkap.2013.51.4.307.
Reference
-
1. Albrektsson T, Brunski J, Wennerberg A. 'A requiem for the periodontal ligament' revisited. Int J Prosthodont. 2009; 22:120–122.2. Xiao SJ, Textor M, Spencer ND, Wieland M, Keller B, Sigrist H. Immobilization of the cell-adhesive peptide Arg-Gly-Asp-Cys (RGDC) on titanium surfaces by covalent chemical attachment. J Mater Sci Mater Med. 1997; 8:867–872.3. Rezania A, Healy KE. The effect of peptide surface density on mineralization of a matrix deposited by osteogenic cells. J Biomed Mater Res. 2000; 52:595–600.4. Bab I, Chorev M. Osteogenic growth peptide: from concept to drug design. Biopolymers. 2002; 66:33–48.5. Tsuchimoto Y, Yoshida Y, Takeuchi M, Mine A, Yatani H, Tagawa Y, Van Meerbeek B, Suzuki K, Kuboki T. Effect of surface pre-treatment on durability of resin-based cements bonded to titanium. Dent Mater. 2006; 22:545–552.6. Jang HW, Kang JK, Lee K, Lee YS, Park PK. A retrospective study on related factors affecting the survival rate of dental implants. J Adv Prosthodont. 2011; 3:204–215.7. Pak HS, Yeo IS, Yang JH. A histomorphometric study of dental implants with different surface characteristics. J Adv Prosthodont. 2010; 2:142–147.8. Nguyen MN, Lebarbe T, Zouani OF, Pichavant L, Durrieu MC, Héroguez V. Impact of RGD nanopatterns grafted onto titanium on osteoblastic cell adhesion. Biomacromolecules. 2012; 13:896–904.9. Reyes CD, Petrie TA, Burns KL, Schwartz Z, García AJ. Biomolecular surface coating to enhance orthopaedic tissue healing and integration. Biomaterials. 2007; 28:3228–3235.10. Hynes RO. Integrins: a family of cell surface receptors. Cell. 1987; 48:549–554.11. Grigoriou V, Shapiro IM, Cavalcanti-Adam EA, Composto RJ, Ducheyne P, Adams CS. Apoptosis and survival of osteoblast-like cells are regulated by surface attachment. J Biol Chem. 2005; 280:1733–1739.12. Hynes RO. Integrins: bidirectional, allosteric signaling machines. Integrins: bidirectional, allosteric signaling machines. Cell. 2002; 110:673–687.13. Reyes CD, Petrie TA, Burns KL, Schwartz Z, García AJ. Biomolecular surface coating to enhance orthopaedic tissue healing and integration. Biomaterials. 2007; 28:3228–3235.14. Hersel U, Dahmen C, Kessler H. RGD modified polymers: biomaterials for stimulated cell adhesion and beyond. Biomaterials. 2003; 24:4385–4415.15. Bagno A, Piovan A, Dettin M, Chiarion A, Brun P, Gambaretto R, Fontana G, Di Bello C, Palù G, Castagliuolo I. Human osteoblast-like cell adhesion on titanium substrates covalently functionalized with synthetic peptides. Bone. 2007; 40:693–699.16. Porté-Durrieu MC, Guillemot F, Pallu S, Labrugère C, Brouillaud B, Bareille R, Amédée J, Barthe N, Dard M, Baquey Ch. Cyclo-(DfKRG) peptide grafting onto Ti-6Al-4V: physical characterization and interest towards human osteoprogenitor cells adhesion. Biomaterials. 2004; 25:4837–4846.17. Mustafa K, Wennerberg A, Wroblewski J, Hultenby K, Lopez BS, Arvidson K. Determining optimal surface roughness of TiO(2) blasted titanium implant material for attachment, proliferation and differentiation of cells derived from human mandibular alveolar bone. Clin Oral Implants Res. 2001; 12:515–525.18. Sul YT, Johansson C, Wennerberg A, Cho LR, Chang BS, Albrektsson T. Optimum surface properties of oxidized implants for reinforcement of osseointegration: surface chemistry, oxide thickness, porosity, roughness, and crystal structure. Int J Oral Maxillofac Implants. 2005; 20:349–359.19. LeBaron RG, Athanasiou KA. Extracellular matrix cell adhesion peptides: functional applications in orthopedic materials. Tissue Eng. 2000; 6:85–103.20. Grzesik WJ, Robey PG. Bone matrix RGD glycoproteins: immunolocalization and interaction with human primary osteoblastic bone cells in vitro. J Bone Miner Res. 1994; 9:487–496.21. Pierschbacher MD, Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature. 1984; 309:30–33.22. Pierschbacher MD, Ruoslahti E. Variants of the cell recognition site of fibronectin that retain attachment-promoting activity. Proc Natl Acad Sci USA. 1984; 81:5985–5988.23. Massia SP, Hubbell JA. Covalently attached GRGD on polymer surfaces promotes biospecific adhesion of mammalian cells. Ann N Y Acad Sci. 1990; 589:261–270.24. Hersel U, Dahmen C, Kessler H. RGD modified polymers: biomaterials for stimulated cell adhesion and beyond. Biomaterials. 2003; 24:4385–4415.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Studies of Enzymes in Hyman Skin Tissue
- Comparison of Clinical Initial Stability of Hydroxy-apatite Coated Implant and Sandblasted, Large-grit and Acid-etched Implant
- The Amino Acid Compositions of Formula for Children with Inherited Metabolic Disorder
- Effects of RGD Protein on the Bone Resorptive Activity of Osteoclast
- Combined effects of rhBMP-2 and rhVEGF coated onto implants on osseointegration: pilot study