Diabetes Metab J.
2011 Aug;35(4):411-417. 10.4093/dmj.2011.35.4.411.
Efficacy of Sitagliptin When Added to Ongoing Therapy in Korean Subjects with Type 2 Diabetes Mellitus
- Affiliations
-
- 1Division of Endocrinology and Metabolism, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. leemk@skku.edu
- KMID: 2281649
- DOI: http://doi.org/10.4093/dmj.2011.35.4.411
Abstract
- BACKGROUND
To evaluate the clinical efficacy of sitagliptin for reducing plasma glucose levels in Korean subjects with type 2 diabetes mellitus during a 14-week treatment period.
METHODS
Our study design involved the addition of 100 mg sitagliptin once-daily to three ongoing combination therapy regimens and changing from glimepiride and metformin to sitagliptin and metformin.
RESULTS
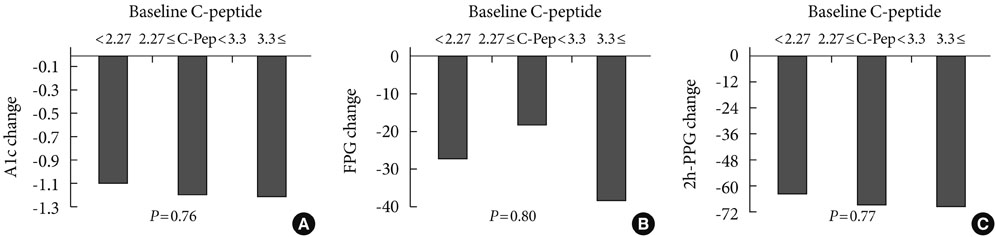
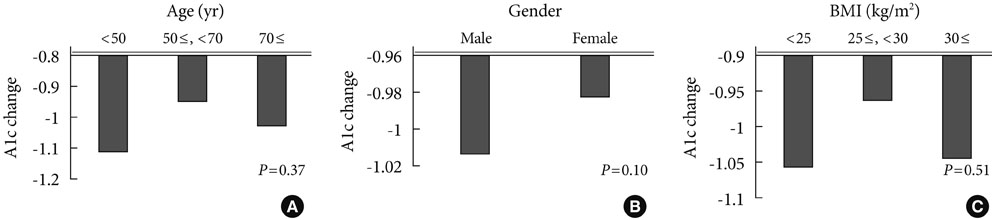
The addition of sitagliptin 100 mg/day produced a statistically significant reduction in mean HbA1c level (mean HbA1c reduction of 0.99+/-0.85%, P<0.01). In the group taking a combination of sitagliptin and metformin (n=143, initial mean HbA1c level=7.48%), the reductions in HbA1c, 2-hour postprandial glucose, and fasting glucose levels were 0.72+/-0.76% (P<0.01), 47+/-65 mg/dL (P<0.01), and 15+/-44 mg/dL (P<0.01), respectively. In the group taking a combination of sitagliptin, glimepiride, and metformin (n=125, initial mean HbA1c level=8.42%), the reductions in HbA1c, 2-hour postprandial glucose, and fasting glucose levels were 1.09+/-0.86% (P<0.01), 62+/-64 mg/dL (P<0.01), and 31+/-45 mg/dL (P<0.01), respectively. In the group taking a combination of sitagliptin, glimepiride, metformin, and alpha-glucosidase inhibitor (n=63, initial mean HbA1c level=9.19%), the reductions in HbA1c, 2-hour postprandial glucose, and fasting glucose levels were 1.27+/-0.70% (P<0.01), 72+/-65 mg/dL (P<0.01), and 35+/-51 mg/dL (P<0.01), respectively. In the group that had previous hypoglycemic events and that changed from glimepiride to sitagliptin, HbA1c level did not change but fasting glucose increased significantly (14+/-29 mg/dL, P<0.01).
CONCLUSION
Sitagliptin combination therapy for 14 weeks significantly improved glycemic control and was well-tolerated in Korean subjects with type 2 diabetes mellitus.
MeSH Terms
Figure
Reference
-
1. Turner RC, Cull CA, Frighi V, Holman RR. UK Prospective Diabetes Study (UKPDS) Group. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus:progressive requirement for multiple therapies (UKPDS 49). JAMA. 1999. 281:2005–2012.2. Hermansen K, Kipnes M, Luo E, Fanurik D, Khatami H, Stein P. Sitagliptin Study 035 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, in patients with type 2 diabetes mellitus inadequately controlled on glimepiride alone or on glimepiride and metformin. Diabetes Obes Metab. 2007. 9:733–745.3. Inzucchi SE. Oral antihyperglycemic therapy for type 2 diabetes: scientific review. JAMA. 2002. 287:360–372.4. Setter SM, Iltz JL, Thams J, Campbell RK. Metformin hydrochloride in the treatment of type 2 diabetes mellitus: a clinical review with a focus on dual therapy. Clin Ther. 2003. 25:2991–3026.5. Hundal RS, Krssak M, Dufour S, Laurent D, Lebon V, Chandramouli V, Inzucchi SE, Schumann WC, Petersen KF, Landau BR, Shulman GI. Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes. 2000. 49:2063–2069.6. Inzucchi SE, Maggs DG, Spollett GR, Page SL, Rife FS, Walton V, Shulman GI. Efficacy and metabolic effects of metformin and troglitazone in type II diabetes mellitus. N Engl J Med. 1998. 338:867–872.7. Doar JW, Thompson ME, Wilde CE, Sewell PF. Diet and oral antidiabetic drugs and plasma sugar and insulin levels in patients with maturity-onset diabetes mellitus. Br Med J. 1976. 1:498–500.8. Iwamoto Y, Tajima N, Kadowaki T, Nonaka K, Taniguchi T, Nishii M, Arjona Ferreira JC, Amatruda JM. Efficacy and safety of sitagliptin monotherapy compared with voglibose in Japanese patients with type 2 diabetes: a randomized, double-blind trial. Diabetes Obes Metab. 2010. 12:613–622.9. Holst JJ, Deacon CF. Inhibition of the activity of dipeptidyl-peptidase IV as a treatment for type 2 diabetes. Diabetes. 1998. 47:1663–1670.10. Bergman AJ, Stevens C, Zhou Y, Yi B, Laethem M, De Smet M, Snyder K, Hilliard D, Tanaka W, Zeng W, Tanen M, Wang AQ, Chen L, Winchell G, Davies MJ, Ramael S, Wagner JA, Herman GA. Pharmacokinetic and pharmacodynamic properties of multiple oral doses of sitagliptin, a dipeptidyl peptidase-IV inhibitor: a double-blind, randomized, placebo-controlled study in healthy male volunteers. Clin Ther. 2006. 28:55–72.11. Herman GA, Bergman A, Stevens C, Kotey P, Yi B, Zhao P, Dietrich B, Golor G, Schrodter A, Keymeulen B, Lasseter KC, Kipnes MS, Snyder K, Hilliard D, Tanen M, Cilissen C, De Smet M, de Lepeleire I, Van Dyck K, Wang AQ, Zeng W, Davies MJ, Tanaka W, Holst JJ, Deacon CF, Gottesdiener KM, Wagner JA. Effect of single oral doses of sitagliptin, a dipeptidyl peptidase-4 inhibitor, on incretin and plasma glucose levels after an oral glucose tolerance test in patients with type 2 diabetes. J Clin Endocrinol Metab. 2006. 91:4612–4619.12. Pitocco D, Zaccardi F, Martini F, Scavone G, Musella T, Caputo S, Ghirlanda G. Severe leucopenia associated with sitagliptin use. Diabetes Res Clin Pract. 2011. 91:e30–e32.13. Scott R, Loeys T, Davies MJ, Engel SS. Sitagliptin Study 801 Group. Efficacy and safety of sitagliptin when added to ongoing metformin therapy in patients with type 2 diabetes. Diabetes Obes Metab. 2008. 10:959–969.14. Wajchenberg BL. Beta-cell failure in diabetes and preservation by clinical treatment. Endocr Rev. 2007. 28:187–218.15. Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003. 52:102–110.16. Maedler K, Carr RD, Bosco D, Zuellig RA, Berney T, Donath MY. Sulfonylurea induced beta-cell apoptosis in cultured human islets. J Clin Endocrinol Metab. 2005. 90:501–506.17. Zhang X, Wang Z, Huang Y, Wang J. Effects of chronic administration of alogliptin on the development of diabetes and beta cell function in high fat diet/streptozotocin diabetic mice. Diabetes Obes Metab. 2011. 13:337–347.18. Gerich J. DPP-4 inhibitors: what may be the clinical differentiators? Diabetes Res Clin Pract. 2010. 90:131–140.19. Yoon KH, Ko SH, Cho JH, Lee JM, Ahn YB, Song KH, Yoo SJ, Kang MI, Cha BY, Lee KW, Son HY, Kang SK, Kim HS, Lee IK, Bonner-Weir S. Selective beta-cell loss and alpha-cell expansion in patients with type 2 diabetes mellitus in Korea. J Clin Endocrinol Metab. 2003. 88:2300–2308.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Letter: Predictive Clinical Parameters for the Therapeutic Efficacy of Sitagliptin in Korean Type 2 Diabetes Mellitus (Diabetes Metab J 2011;35:159-65)
- Predictive Clinical Parameters for the Therapeutic Efficacy of Sitagliptin in Korean Type 2 Diabetes Mellitus
- Effects of Sitagliptin on Insulin and Glucagon Levels in Type 2 Diabetes Mellitus
- Response: Predictive Clinical Parameters for the Therapeutic Efficacy of Sitagliptin in Korean Type 2 Diabetes Mellitus (Diabetes Metab J 2011;35:159-65)
- Effects of 6-Month Sitagliptin Treatment on Insulin and Glucagon Responses in Korean Patients with Type 2 Diabetes Mellitus