Diabetes Metab J.
2011 Feb;35(1):41-49. 10.4093/dmj.2011.35.1.41.
Basal C-peptide Level as a Surrogate Marker of Subclinical Atherosclerosis in Type 2 Diabetic Patients
- Affiliations
-
- 1Department of Internal Medicine, Konyang University Hospital, Konyang University School of Medicine, Daejon, Korea. mdldm@hanmail.net
- 2Konyang University Myunggok Medical Research Institute, Daejon, Korea.
- 3Department of Internal Medicine, Jeju National University Hospital, Jeju National University School of Medicine, Jeju, Korea.
- KMID: 2281491
- DOI: http://doi.org/10.4093/dmj.2011.35.1.41
Abstract
- BACKGROUND
Recent studies have revealed that C-peptide induces smooth muscle cell proliferation and causes human atherosclerotic lesions in diabetic patients. The present study was designed to examine whether the basal C-peptide levels correlate with cardiovascular risk in type 2 diabetes mellitus (T2DM) patients.
METHODS
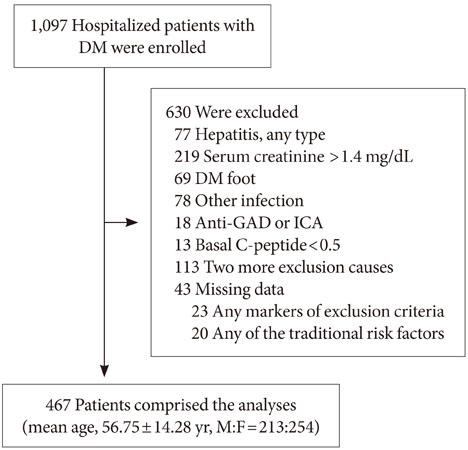
Data was obtained from 467 patients with T2DM from two institutions who were followed for four years. The medical findings of all patients were reviewed, and patients with creatinine >1.4 mg/dL, any inflammation or infection, hepatitis, or type 1 DM were excluded. The relationships between basal C-peptide and other clinical values were statistically analyzed.
RESULTS
A simple correlation was found between basal C-peptide and components of metabolic syndrome (MS). Statistically basal C-peptide levels were significantly higher than the three different MS criteria used in the present study, the Adult Treatment Panel III (ATP III) of the National Cholesterol Education Program's (NCEP's), World Health Organization (WHO), and the International Diabetes Federation (IDF) criteria (NCEP-ATP III, P=0.001; IDF, P<0.001; WHO, P=0.029). The multiple regression analysis between intima-media thickness (IMT) and clinical values showed that basal C-peptide significantly correlated with IMT (P=0.043), while the analysis between the 10-year coronary heart disease risk by the United Kingdom Prospective Diabetes Study risk engine and clinical values showed that basal C-peptide did not correlate with IMT (P=0.226).
CONCLUSION
Basal C-peptide is related to cardiovascular predictors (IMT) of T2DM, suggesting that basal C-peptide does provide a further indication of cardiovascular disease.
MeSH Terms
Figure
Reference
-
1. Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myo cardial infarction. N Engl J Med. 1998. 339:229–234.2. Ridker PM, Brown NJ, Vaughan DE, Harrison DG, Mehta JL. Established and emerging plasma biomarkers in the prediction of first atherothrombotic events. Circulation. 2004. 109:25 Suppl 1. IV6–IV19.3. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 2003. 26:Suppl 1. S5–S20.4. Air EL, Kissela BM. Diabetes, the metabolic syndrome, and ischemic stroke: epidemiology and possible mechanisms. Diabetes Care. 2007. 30:3131–3140.5. Clark PM, Hales CN. How to measure plasma insulin. Diabetes Metab Rev. 1994. 10:79–90.6. O'Rahilly S, Burnett MA, Smith RF, Darley JH, Turner RC. Haemolysis affects insulin but not C-peptide immunoassay. Diabetologia. 1987. 30:394–396.7. Haban P, Simoncic R, Zidekova E, Ozdin L. Role of fasting serum C-peptide as a predictor of cardiovascular risk associated with the metabolic X-syndrome. Med Sci Monit. 2002. 8:CR175–CR179.8. Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998. 15:539–553.9. Denke MA, Pasternak RC. Defining and treating the metabolic syndrome: a primer from the Adult Treatment Panel III. Curr Treat Options Cardiovasc Med. 2001. 3:251–253.10. Alberti KG, Zimmet P, Shaw J. IDF Epidemiology Task Force Consensus Group. The metabolic syndrome--a new worldwide definition. Lancet. 2005. 366:1059–1062.11. Kitabchi AE. Proinsulin and C-peptide: a review. Metabolism. 1977. 26:547–587.12. Rigler R, Pramanik A, Jonasson P, Kratz G, Jansson OT, Nygren P, Stahl S, Ekberg K, Johansson B, Uhlen S, Uhlen M, Jornvall H, Wahren J. Specific binding of proinsulin C-peptide to human cell membranes. Proc Natl Acad Sci U S A. 1999. 96:13318–13323.13. Johansson J, Ekberg K, Shafqat J, Henriksson M, Chibalin A, Wahren J, Jornvall H. Molecular effects of proinsulin C-peptide. Biochem Biophys Res Commun. 2002. 295:1035–1040.14. Nordquist L, Johansson M. Proinsulin C-peptide: friend or foe in the development of diabetes-associated complications? Vasc Health Risk Manag. 2008. 4:1283–1288.15. Schlessinger J. New roles for Src kinases in control of cell survival and angiogenesis. Cell. 2000. 100:293–296.16. Cho M, Park JS, Nam J, Kim CS, Nam JH, Kim HJ, Ahn CW, Cha BS, Lim SK, Kim KR, Lee HC, Huh KB. Association of abdominal obesity with atherosclerosis in type 2 diabetes mellitus (T2DM) in Korea. J Korean Med Sci. 2008. 23:781–788.17. Manolio TA, Savage PJ, Burke GL, Liu KA, Wagenknecht LE, Sidney S, Jacobs DR Jr, Roseman JM, Donahue RP, Oberman A. Association of fasting insulin with blood pressure and lipids in young adults. The CARDIA study. Arteriosclerosis. 1990. 10:430–436.18. Chen CH, Tsai ST, Chou P. Correlation of fasting serum C-peptide and insulin with markers of metabolic syndrome-X in a homogenous Chinese population with normal glucose tolerance. Int J Cardiol. 1999. 68:179–186.19. Orchard TJ, Eichner J, Kuller LH, Becker DJ, McCallum LM, Grandits GA. Insulin as a predictor of coronary heart disease: interaction with apolipoprotein E phenotype. A report from the Multiple Risk Factor Intervention Trial. Ann Epidemiol. 1994. 4:40–45.20. Rubins HB, Robins SJ, Collins D, Fye CL, Anderson JW, Elam MB, Faas FH, Linares E, Schaefer EJ, Schectman G, Wilt TJ, Wittes J. Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. N Engl J Med. 1999. 341:410–418.21. Howard G, O'Leary DH, Zaccaro D, Haffner S, Rewers M, Hamman R, Selby JV, Saad MF, Savage P, Bergman R. Insulin sensitivity and atherosclerosis. The Insulin Resistance Atherosclerosis Study (IRAS) Investigators. Circulation. 1996. 93:1809–1817.22. Semenkovich CF. Insulin resistance and atherosclerosis. J Clin Invest. 2006. 116:1813–1822.23. Park SH, Marso SP, Zhou Z, Foroudi F, Topol EJ, Lincoff AM. Neointimal hyperplasia after arterial injury is increased in a rat model of non-insulin-dependent diabetes mellitus. Circulation. 2001. 104:815–819.24. The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993. 329:977–986.25. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998. 352:837–853.26. Marx N, Walcher D, Raichle C, Aleksic M, Bach H, Grub M, Hombach V, Libby P, Zieske A, Homma S, Strong J. C-peptide colocalizes with macrophages in early arteriosclerotic lesions of diabetic subjects and induces monocyte chemotaxis in vitro. Arterioscler Thromb Vasc Biol. 2004. 24:540–545.27. Wang CC, Goalstone ML, Draznin B. Molecular mechanisms of insulin resistance that impact cardiovascular biology. Diabetes. 2004. 53:2735–2740.28. Bruemmer D. C-Peptide in insulin resistance and vascular complications: teaching an old dog new tricks. Circ Res. 2006. 99:1149–1151.29. Lonn E. Carotid artery intima-media thickness: a new noninvasive gold standard for assessing the anatomic extent of atherosclerosis and cardiovascular risk? Clin Invest Med. 1999. 22:158–160.30. Folsom AR, Eckfeldt JH, Weitzman S, Ma J, Chambless LE, Barnes RW, Cram KB, Hutchinson RG. Relation of carotid artery wall thickness to diabetes mellitus, fasting glucose and insulin, body size, and physical activity. Atherosclerosis Risk in Communities (ARIC) Study Investigators. Stroke. 1994. 25:66–73.31. Hirai FE, Moss SE, Klein BE, Klein R. Relationship of glycemic control, exogenous insulin, and C-peptide levels to ischemic heart disease mortality over a 16-year period in people with older-onset diabetes: the Wisconsin Epidemiologic Study of Diabetic Retinopathy (WESDR). Diabetes Care. 2008. 31:493–497.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Letter: Basal C-peptide Level as a Surrogate Marker of Subclinical Atherosclerosis in Type 2 Diabetes Patients (Diabetes Metab J 2011;35:41-9)
- Response: Basal C-peptide Level as a Surrogate Marker of Subclinical Atherosclerosis in Type 2 Diabetic Patients (Diabetes Metab J 2011;35:41-9)
- Cardio-Ankle Vascular Index as a Surrogate Marker of Early Atherosclerotic Cardiovascular Disease in Koreans with Type 2 Diabetes Mellitus
- Subclinical Atherosclerosis in Patients with Cushing Syndrome: Evaluation with Carotid Intima-Media Thickness and Ankle-Brachial Index
- Non-Alcoholic Fatty Liver Disease, a Marker of Subclinical Atherosclerosis Applicable Only to Metabolic Syndrome?: Time to Organize the Connection between Metabolism and Atherosclerosis