Korean J Perinatol.
2013 Jun;24(2):95-100. 10.14734/kjp.2013.24.2.95.
A Case of Term Delivery with Diagnosis of Severe Oligohydramnios after Exposure to Glimepiride, Metformin and Antihypertensive agents Including Angiotensin Receptor Antagonist up to Approximately 20 Weeks of Pregnancy
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Cheil General Hospital and Women's Healthcare Center, Seoul, Korea. kdw1015@gmail.com
- 2Department of Pediatrics, Mizmedi Hospital, Seoul, Korea.
- KMID: 2280926
- DOI: http://doi.org/10.14734/kjp.2013.24.2.95
Abstract
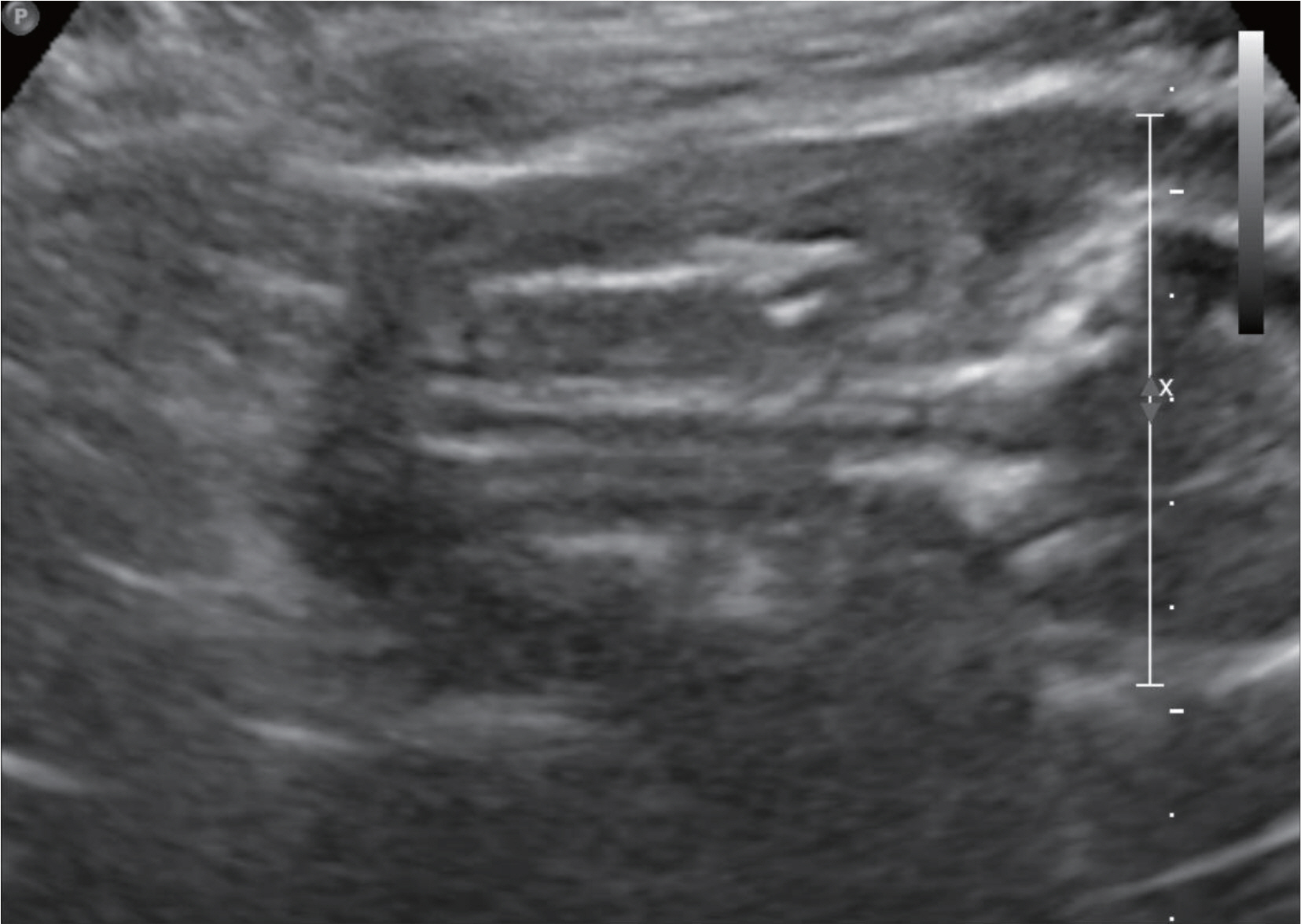
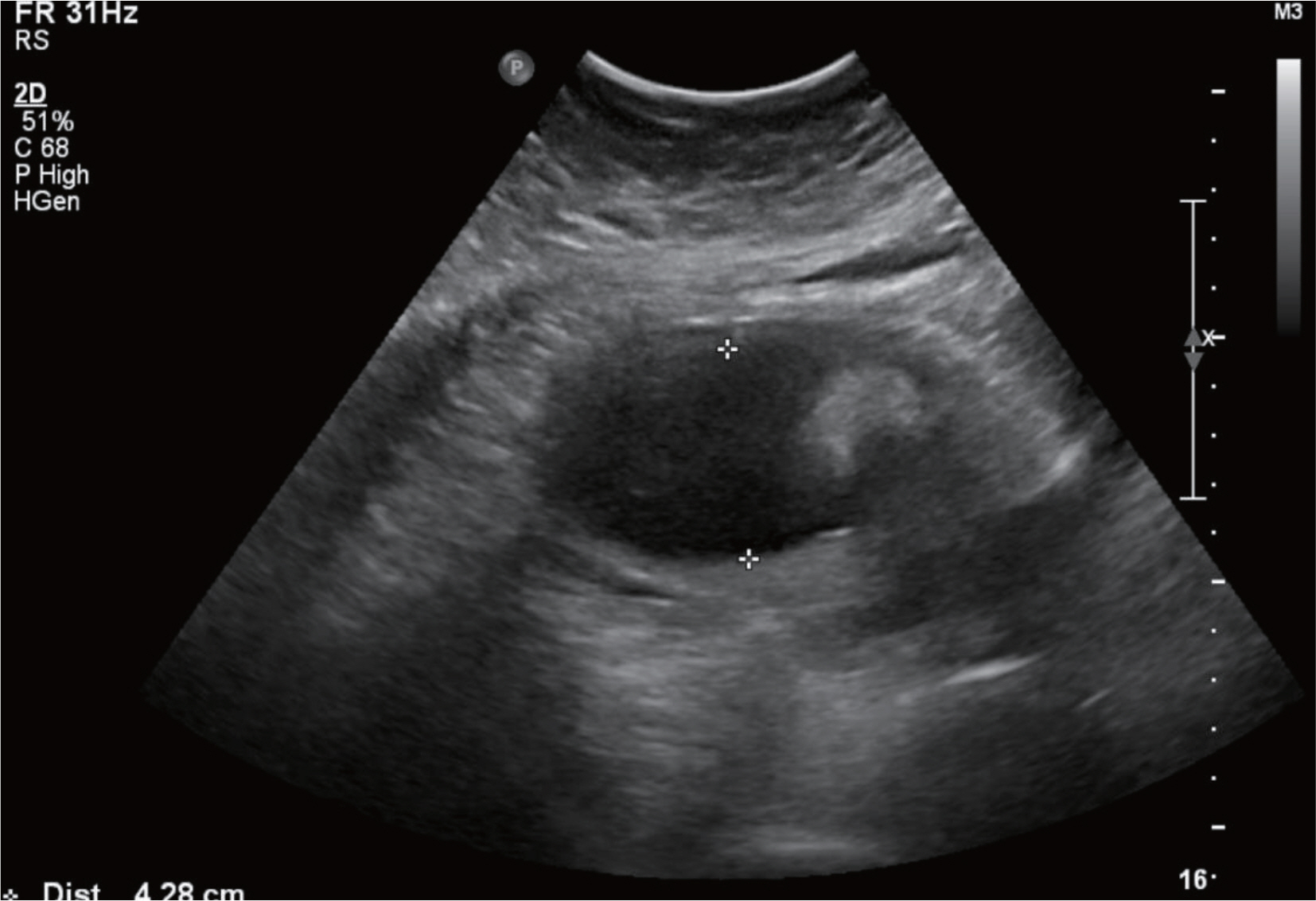
- Various fetal and maternal conditions are known to be associated with oligohydramnios. In general, oligohydramnios developed early in pregnancy is less common but frequently has a poor prognosis. The use of angiotensin-converting enzyme inhibitors or angiotensin receptor antagonists during pregnancy has been associated with oligohydramnios as well as growth restriction, pulmonary hypoplasia with respiratory distress, acute renal failure, cranial malformation and fetal death. Although many researchers report a use of oral hypoglycemic agents such as glyburide or metformin in gestational diabetes mellitus, but potential adverse effects of glimepiride, which is relatively recently developed, is not well known owing to the lack of clinical data, especially early in pregnancy. A 41-year-old woman with chronic hypertension and type 2 diabetes mellitus was treated with drugs including metformin, glimepiride and angiotensin receptor antagonist until approximately 20 weeks' gestations, when severe oligohydramnios was noted. After the hospitalization for bed rest, fetal surveillance, and discontinuation of the agents, amniotic fluid reaccumulated, and the infant was delivered at term. We report this case with a brief review of literatures.
Keyword
MeSH Terms
-
Acute Kidney Injury
Amniotic Fluid
Angiotensin Receptor Antagonists
Angiotensin-Converting Enzyme Inhibitors
Angiotensins
Antihypertensive Agents
Bed Rest
Diabetes Mellitus
Diabetes Mellitus, Type 2
Diabetes, Gestational
Female
Fetal Death
Glyburide
Hospitalization
Humans
Hypertension
Hypoglycemic Agents
Infant
Metformin
Oligohydramnios
Pregnancy
Prognosis
Sulfonylurea Compounds
Angiotensin Receptor Antagonists
Angiotensin-Converting Enzyme Inhibitors
Angiotensins
Antihypertensive Agents
Glyburide
Hypoglycemic Agents
Metformin
Sulfonylurea Compounds
Figure
Reference
-
1). Pryde PG., Sedman AB., Nugent CE., Barr M Jr. Angiotensin-converting enzyme inhibitor fetopathy. J Am Soc Nephrol. 1993. 3:1575–82.
Article2). Bullo M., Tschumi S., Bucher BS., Bianchetti MG., Simonetti GD. Pregnancy outcome following exposure to angiotensin-converting enzyme inhibitors or angiotensin receptor antagonists: a systematic review. Hypertension. 2012. 60:444–50.3). Langer O., Conway DL., Berkus MD., Xenakis EM., Gonzales O. A comparison of glyburide and insulin in women with gestational diabetes mellitus. N Engl J Med. 2000. 343:1134–8.
Article4). Nicholson W., Baptiste-Roberts K. Oral hypoglycaemic agents during pregnancy: The evidence for effectiveness and safety. Best Practice & Research Clinical Obstet Gynaecol. 2011. 25:51–63.
Article5). Vanky E., Stridsklev S., Heimstad R., Romundstad P., Skogøy K., Kleggetveit O, et al. Metformin versus placebo from first trimester to delivery in polycystic ovary syndrome: a randomized, controlled multicenter study. J Clin Endocrinol Metab. 2010. 95:448–55.
Article6). Piacquadio K., Hollingsworth DR., Murphy H. Effects of in-utero exposure to oral hypoglycaemic drugs. Lancet. 1991. 338:866–9.
Article7). American College of Obstetricians and Gynecologists: Gestational diabetes. Obstet Gynecol. 2001. 98:525–38.8). Kraus GW., Marchese JR., Yen SS. Prophylactic use of hydrochlorothiazide in pregnancy. JAMA. 1966. 198:1150–4.
Article9). Ahn HK., Nava-Ocampo AA., Han JY., Choi JS., Chung JH., Yang JH, et al. Exposure to amlodipine in the first trimester of pregnancy and during breastfeeding. Hypertens Pregnancy. 2007. 26:179–87.
Article10). Cooper WO., Hernandez-Diaz S., Arbogast PG., Dudley JA., Dyer S., Gideon PS, et al. Major congenital malformations after first-trimester exposure to ACE inhibitors. N Engl J Med. 2006. 354:2443–51.
Article11). Li DK., Yang C., Andrade S., Tavares V., Ferber JR. Maternal exposure to angiotensin converting enzyme inhibitors in the first trimester and risk of malformations in offspring: a retrospective cohort study. BMJ. 2011. 343:d5931.
Article12). Chisholm CA., Chescheir NC., Kennedy M. Reversible oligohydramnios in a pregnancy with angiotensin-converting enzyme inhibitor exposure. Am J Perinatol. 1997. 14:511–3.
Article13). Muller PR., James A. Pregnancy with prolonged fetal exposure to an angiotensin-converting enzyme inhibitor. J Perinatol. 2002. 22:582–4.
Article14). Celentano C., Prefumo F., di Vera E., Iannicco A., Gallo DP., Liberati M. Reversible acute fetal renal failure due to maternal exposure to angiotensin receptor blocker. Pediatr Nephol. 2008. 23:333–4.
Article15). Hanssens M., Keirse MJ., Vankelecom F., Van Assche FA. Fetal and neonatal effects of treatment with angiotensin-converting enzyme inhibitors in pregnancy. Obstet Gynecol. 1991. 78:128–35.16). Cooke CE., Fatodu H. Physician conformity and patient adherence to ACE inhibitors and ARBs in patients with diabetes, with and without renal disease and hypertension, in a medicaid managed care organization. J Manag Care Pharm. 2006. 12:649–55.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Extracorporeal Membrane Oxygenation and Continuous Renal Replacement Therapy for Treatment of Calcium Channel Blockers, Angiotensin II Receptor Blockers, and Metformin Overdose
- Hypocalvaria of Newborn Infant: Intrauterine Exposure to an Angiotensin Receptor Blocker
- A Pregnant Woman with Type 2 Diabetes Unintentionally Exposed to Metformin and Voglibose until the Second Trimester of Pregnancy: A Case Report
- A Case of Neonate with Acute Renal Failure after Maternal Treatment with Angiotensin II Receptor Blocker
- New drugs for treatment of hypertension