Clin Nutr Res.
2015 Apr;4(2):132-136. 10.7762/cnr.2015.4.2.132.
Nutrition Therapy for Mitochondrial Neurogastrointestinal Encephalopathy with Homozygous Mutation of the TYMP Gene
- Affiliations
-
- 1Department of Clinical Nutrition, Nanjing Drum Tower Hospital, The Affiliated Hospital of Nanjing University Medical School, Nanjing 210008, China.
- 2Department of Parenteral and Enteral, Peking Union Medical School Hospital, Beijing 100730, China. txchenwei@sina.com
- 3Department of Public Health, Food Study and Nutrition, Syracuse University, Syracuse, NY 13244, USA.
- 4Department of Gastroenterology, Peking Union Medical School Hospital, Beijing 100730, China.
- KMID: 2279359
- DOI: http://doi.org/10.7762/cnr.2015.4.2.132
Abstract
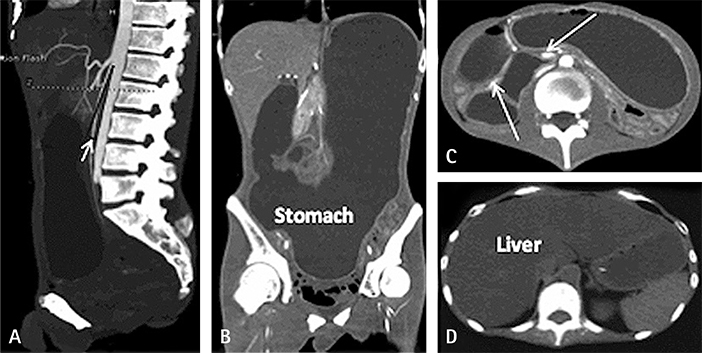
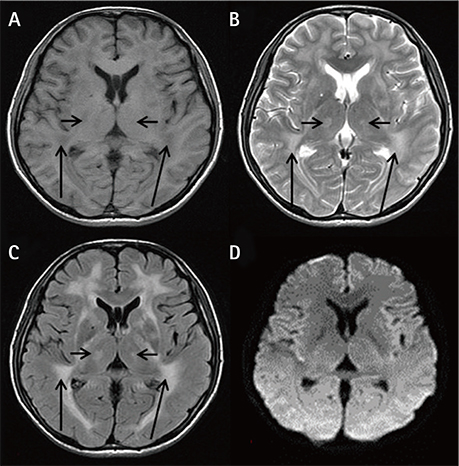
- Mitochondrial neurogastrointestinal encephalopathy (MNGIE) is characterized by significant gastrointestinal dysmotility. Early and long-term nutritional therapy is highly recommended. We report a case of MNGIE in a patient who was undergoing long-term nutrition therapy. He was diagnosed with a serious symptom of fatty liver and hyperlipidemia complications, along with homozygous mutation of the thymidine phosphorylase (TYMP) gene (c.217G > A). To our knowledge, this is the first report of such a case. Herein, we describe preventive measures for the aforementioned complications and mitochondrial disease-specific nutritional therapy.
Keyword
MeSH Terms
Figure
Reference
-
1. Garone C, Tadesse S, Hirano M. Clinical and genetic spectrum of mitochondrial neurogastrointestinal encephalomyopathy. Brain. 2011; 134:3326–3332.
Article2. Xieng Y, Gu WY, Hou XL, Yang YL, Jiang Y, Zhou CL. Mitochondrial hyneurogastrointestinal encephalomyopathy of neonatal onset: a case report and literature review. Chin J Evid Based Pediatr. 2013; 8:126–130.3. Yavuz H, Ozel A, Christensen M, Christensen E, Schwartz M, Elmaci M, Vissing J. Treatment of mitochondrial hyneurogastrointestinal encephalomyopathy with dialysis. Arch Neurol. 2007; 64:435–438.
Article4. Hartl WH, Jauch KW, Parhofer K, Rittler P. Working group for developing the guidelines for parenteral nutrition of the German Association for Nutritional Medicine. Complications and monitoring - Guidelines on Parenteral Nutrition, Chapter 11. Ger Med Sci. 2009; 7:Doc17.5. Cavicchi M, Beau P, Crenn P, Degott C, Messing B. Prevalence of liver disease and contributing factors in patients receiving home parenteral nutrition for permanent intestinal failure. Ann Intern Med. 2000; 132:525–532.
Article6. Singer P, Berger MM, Van den Berghe G, Biolo G, Calder P, Forbes A, Griffiths R, Kreyman G, Leverve X, Pichard C. Espen. ESPEN Guidelines on Parenteral Nutrition: intensive care. Clin Nutr. 2009; 28:387–400.
Article7. Xu ZW, Li YS. Pathogenesis and treatment of parenteral nutrition-associated liver disease. Hepatobiliary Pancreat Dis Int. 2012; 11:586–593.
Article8. Rubin M, Moser A, Vaserberg N, Greig F, Levy Y, Spivak H, Ziv Y, Lelcuk S. Structured triacylglycerol emulsion, containing both medium- and long-chain fatty acids, in long-term home parenteral nutrition: a double-blind randomized cross-over study. Nutrition. 2000; 16:95–100.
Article9. Allen RJ, DiMauro S, Coulter DL, Papadimitriou A, Rothenberg SP. Kearns-Sayre syndrome with reduced plasma and cerebrospinal fluid folate. Ann Neurol. 1983; 13:679–682.
Article10. Melegh B, Seress L, Bedekovics T, Kispál G, Sümegi B, Trombitás K, Méhes K. Muscle carnitine acetyltransferase and carnitine deficiency in a case of mitochondrial encephalomyopathy. J Inherit Metab Dis. 1999; 22:827–838.
Article11. Artuch R, Vilaseca MA, Pineda M. Biochemical monitoring of the treatment in paediatric patients with mitochondrial disease. J Inherit Metab Dis. 1998; 21:837–845.
Article12. Pichard C, Mühlebach S, Maisonneuve N, Sierro C. Prospective survey of parenteral nutrition in Switzerland: a three-year nation-wide survey. Clin Nutr. 2001; 20:345–350.
Article13. Staun M, Pironi L, Bozzetti F, Baxter J, Forbes A, Joly F, Jeppesen P, Moreno J, Hébuterne X, Pertkiewicz M, Mühlebach S, Shenkin A, Van Gossum A. ESPEN. ESPEN Guidelines on Parenteral Nutrition: home parenteral nutrition (HPN) in adult patients. Clin Nutr. 2009; 28:467–479.
Article14. Napolitano A, Salvetti S, Vista M, Lombardi V, Siciliano G, Giraldi C. Long-term treatment with idebenone and riboflavin in a patient with MELAS. Neurol Sci. 2000; 21:S981–S982.
Article15. Bakker HD, Scholte HR, Jeneson JA. Vitamin E in a mitochondrial myopathy with proliferating mitochondria. Lancet. 1993; 342:175–176.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Mitochondrial Intestinal Pseudo-Obstruction with Neurogenic Bladder Syndrome: Point Mutation at T8356C: A New Mitochondrial Disease?
- Mutation Analyses in Korean patients with MELAS (Mitochondrial Encephalomyopathy, Lactic acidosis, and Stroke like episodes)
- Hereditary spastic paraplegia with thin corpus callosum due to novel homozygous mutation in SPG11 gene
- Point Mutations in a Mitochondrial Transfer RiboNucleic Acid Gene in South Korean Women with Preeclampsia
- A homozygous mutation in the insulin gene (INS) causing autosomal recessive neonatal diabetes in Saudi families