Clin Exp Otorhinolaryngol.
2013 Jun;6(2):99-102.
Syndromic Hearing Loss in Association with PTPN11-Related Disorder: The Experience of Cochlear Implantation in a Child with LEOPARD Syndrome
- Affiliations
-
- 1Department of Otorhinolaryngology-Head and Neck Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. hongsh@skku.edu
- 2Department of Laboratory Medicine & Genetics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. heejinkim@skku.edu
Abstract
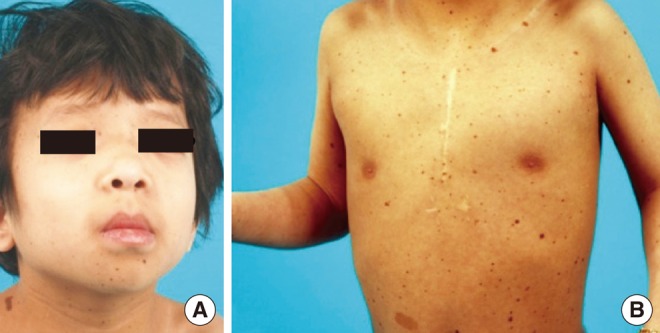
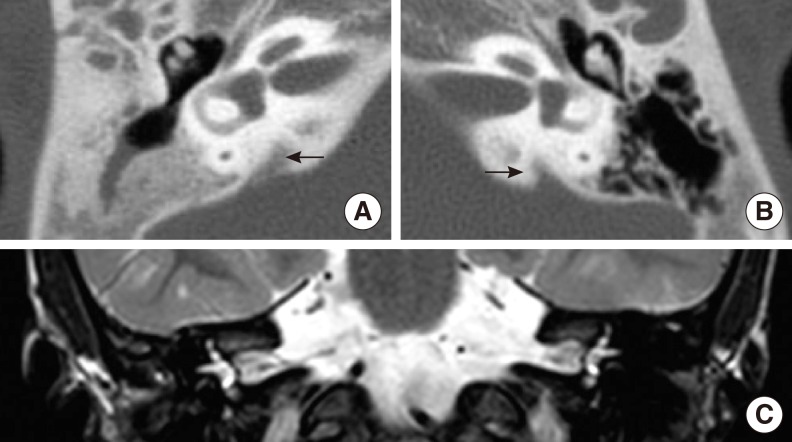
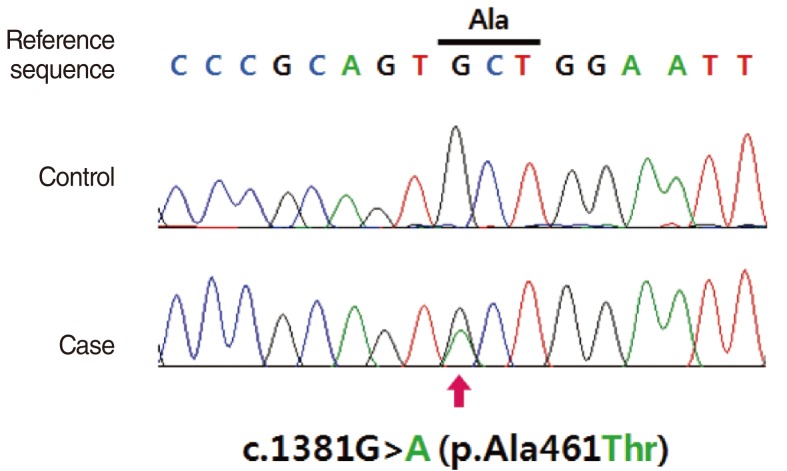
- Hearing loss (HL) is one of the most frequent clinical manifestations of patients who suffer with multi-systemic genetic disorders. HL in association with other physical stigmata is referred to as a syndromic form of HL. LEOPARD syndrome (LS) is one of the disorders with syndromic HL and it is caused by a mutation in the PTPN11 or RAF1 gene. In general, 5 year old children who undergo cochlear implantation usually show a marked change in behavior regarding sound detection within the first 6 months of implant use, but word identification may not be exhibited for at least another 6-12 months of implant use. We herein report on a 5-year-old girl with LS. Her clinical manifestations including bilateral sensorineural HL, which indicated the diagnosis of LS. We confirmed the diagnosis by identifying a disease-causing mutation in the PTPN11 gene, which was a heterozygous missense mutation Ala461Thr (c.1381G>A). She underwent cochlear implantation (CI) without complications and she is currently on regular follow-up at postoperative 1 year. This is the first reported case of CI in a patient with LS in the medical literature.
Keyword
MeSH Terms
Figure
Reference
-
1. Gorlin RJ, Anderson RC, Blaw M. Multiple lentigenes syndrome. Am J Dis Child. 1969; 6. 117(6):652–662. PMID: 5771505.
Article2. Seuanez H, Mane-Garzon F, Kolski R. Cardio-cutaneous syndrome (the "LEOPARD" syndrome): review of the literature and a new family. Clin Genet. 1976; 3. 9(3):266–276. PMID: 1261064.
Article3. Digilio MC, Conti E, Sarkozy A, Mingarelli R, Dottorini T, Marino B, et al. Grouping of multiple-lentigines/LEOPARD and Noonan syndromes on the PTPN11 gene. Am J Hum Genet. 2002; 8. 71(2):389–394. PMID: 12058348.
Article4. Legius E, Schrander-Stumpel C, Schollen E, Pulles-Heintzberger C, Gewillig M, Fryns JP. PTPN11 mutations in LEOPARD syndrome. J Med Genet. 2002; 8. 39(8):571–574. PMID: 12161596.
Article5. Pandit B, Sarkozy A, Pennacchio LA, Carta C, Oishi K, Martinelli S, et al. Gain-of-function RAF1 mutations cause Noonan and LEOPARD syndromes with hypertrophic cardiomyopathy. Nat Genet. 2007; 8. 39(8):1007–1012. PMID: 17603483.
Article6. Voron DA, Hatfield HH, Kalkhoff RK. Multiple lentigines syndrome: case report and review of the literature. Am J Med. 1976; 3. 60(3):447–456. PMID: 1258892.7. Allanson JE, Hall JG, Van Allen MI. Noonan phenotype associated with neurofibromatosis. Am J Med Genet. 1985; 7. 21(3):457–462. PMID: 2411134.
Article8. Ahlbom BE, Dahl N, Zetterqvist P, Anneren G. Noonan syndrome with cafe-au-lait spots and multiple lentigines syndrome are not linked to the neurofibromatosis type 1 locus. Clin Genet. 1995; 8. 48(2):85–89. PMID: 7586657.9. Colley A, Donnai D, Evans DG. Neurofibromatosis/Noonan phenotype: a variable feature of type 1 neurofibromatosis. Clin Genet. 1996; 2. 49(2):59–64. PMID: 8740913.
Article10. Coppin BD, Temple IK. Multiple lentigines syndrome (LEOPARD syndrome or progressive cardiomyopathic lentiginosis). J Med Genet. 1997; 7. 34(7):582–586. PMID: 9222968.
Article11. Limongelli G, Pacileo G, Marino B, Digilio MC, Sarkozy A, Elliott P, et al. Prevalence and clinical significance of cardiovascular abnormalities in patients with the LEOPARD syndrome. Am J Cardiol. 2007; 8. 100(4):736–741. PMID: 17697839.
Article12. Limongelli G, Sarkozy A, Pacileo G, Calabro P, Digilio MC, Maddaloni V, et al. Genotype-phenotype analysis and natural history of left ventricular hypertrophy in LEOPARD syndrome. Am J Med Genet A. 2008; 3. 146A(5):620–628. PMID: 18241070.
Article13. Sarkozy A, Conti E, Digilio MC, Marino B, Morini E, Pacileo G, et al. Clinical and molecular analysis of 30 patients with multiple lentigines LEOPARD syndrome. J Med Genet. 2004; 5. 41(5):e68. PMID: 15121796.
Article14. Sarkozy A, Obregon MG, Conti E, Esposito G, Mingarelli R, Pizzuti A, et al. A novel PTPN11 gene mutation bridges Noonan syndrome, multiple lentigines/LEOPARD syndrome and Noonan-like/multiple giant cell lesion syndrome. Eur J Hum Genet. 2004; 12. 12(12):1069–1072. PMID: 15470362.15. Staller SJ, Beiter AL, Brimacombe JA. Use of the Nucleus 22 channel cochlear implant system with children. Volta Rev. 1994; 96:15–40.