Korean J Pain.
2015 Jan;28(1):4-10. 10.3344/kjp.2015.28.1.4.
Etifoxine for Pain Patients with Anxiety
- Affiliations
-
- 1Department of Anesthesia and Pain Medicine, School of Medicine, Pusan National University, Yangsan, Korea. pain@pusan.ac.kr
- KMID: 2278247
- DOI: http://doi.org/10.3344/kjp.2015.28.1.4
Abstract
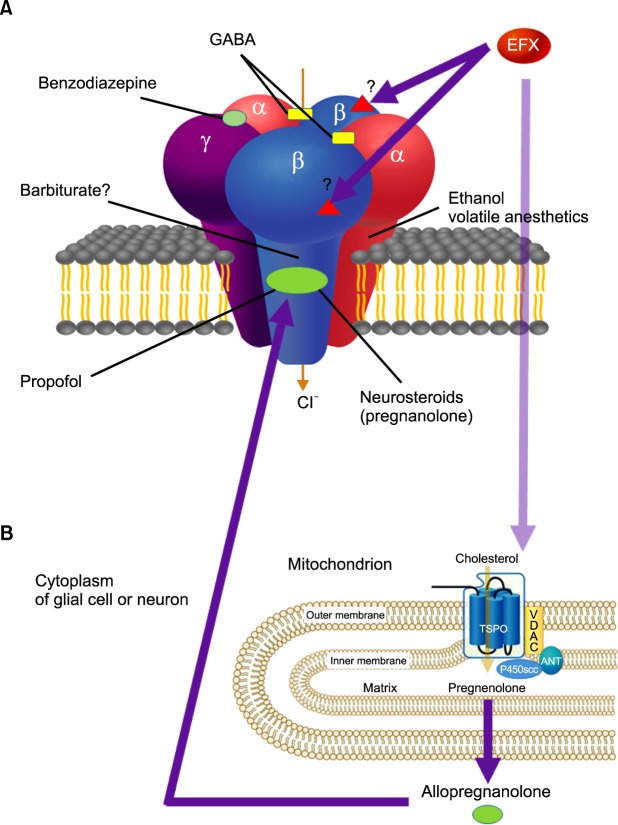
- Etifoxine (etafenoxine, Stresam(R)) is a non-benzodiazepine anxiolytic with an anticonvulsant effect. It was developed in the 1960s for anxiety disorders and is currently being studied for its ability to promote peripheral nerve healing and to treat chemotherapy-induced pain. In addition to being mediated by GABA(A)alpha2 receptors like benzodiazepines, etifoxine appears to produce anxiolytic effects directly by binding to beta2 or beta3 subunits of the GABA(A) receptor complex. It also modulates GABA(A) receptors indirectly via stimulation of neurosteroid production after etifoxine binds to the 18 kDa translocator protein (TSPO) of the outer mitochondrial membrane in the central and peripheral nervous systems, previously known as the peripheral benzodiazepine receptor (PBR). Therefore, the effects of etifoxine are not completely reversed by the benzodiazepine antagonist flumazenil. Etifoxine is used for various emotional and bodily reactions followed by anxiety. It is contraindicated in situations such as shock, severely impaired liver or kidney function, and severe respiratory failure. The average dosage is 150 mg per day for no more than 12 weeks. The most common adverse effect is drowsiness at the initial stage. It does not usually cause any withdrawal syndromes. In conclusion, etifoxine shows less adverse effects of anterograde amnesia, sedation, impaired psychomotor performance, and withdrawal syndromes than those of benzodiazepines. It potentiates GABA(A) receptor-function by a direct allosteric effect and by an indirect mechanism involving the activation of TSPO. It seems promising that non-benzodiazepine anxiolytics including etifoxine will replenish shortcomings of benzodiazepines and selective serotonin reuptake inhibitors according to animated studies related to TSPO.
Keyword
MeSH Terms
-
Amnesia, Anterograde
Anti-Anxiety Agents
Anticonvulsants
Anxiety Disorders
Anxiety*
Benzodiazepines
Flumazenil
Humans
Kidney
Liver
Mitochondrial Membranes
Nerve Regeneration
Neuralgia
Neurotransmitter Agents
Peripheral Nerves
Peripheral Nervous System
Psychomotor Performance
Receptors, GABA-A
Respiratory Insufficiency
Serotonin Uptake Inhibitors
Shock
Sleep Stages
Anti-Anxiety Agents
Anticonvulsants
Benzodiazepines
Flumazenil
Neurotransmitter Agents
Receptors, GABA-A
Serotonin Uptake Inhibitors
Figure
Cited by 1 articles
-
All about pain pharmacology: what pain physicians should know
Kyung-Hoon Kim, Hyo-Jung Seo, Salahadin Abdi, Billy Huh
Korean J Pain. 2020;33(2):108-120. doi: 10.3344/kjp.2020.33.2.108.
Reference
-
1. Means-Christensen AJ, Roy-Byrne PP, Sherbourne CD, Craske MG, Stein MB. Relationships among pain, anxiety, and depression in primary care. Depress Anxiety. 2008; 25:593–600. PMID: 17932958.
Article2. Farb DH, Ratner MH. Targeting the modulation of neural circuitry for the treatment of anxiety disorders. Pharmacol Rev. 2014; 66:1002–1032. PMID: 25237115.
Article3. Almeida TF, Roizenblatt S, Tufik S. Afferent pain pathways: a neuroanatomical review. Brain Res. 2004; 1000:40–56. PMID: 15053950.
Article4. Willis WD, Westlund KN. Neuroanatomy of the pain system and of the pathways that modulate pain. J Clin Neurophysiol. 1997; 14:2–31. PMID: 9013357.
Article5. Price DD. Central neural mechanisms that interrelate sensory and affective dimensions of pain. Mol Interv. 2002; 2:392–403. 339PMID: 14993415.
Article6. Price DD. Psychological and neural mechanisms of the affective dimension of pain. Science. 2000; 288:1769–1772. PMID: 10846154.
Article7. Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science. 1997; 277:968–971. PMID: 9252330.
Article8. Hamon A, Morel A, Hue B, Verleye M, Gillardin JM. The modulatory effects of the anxiolytic etifoxine on GABA(A) receptors are mediated by the beta subunit. Neuropharmacology. 2003; 45:293–303. PMID: 12871647.
Article9. Schlichter R, Rybalchenko V, Poisbeau P, Verleye M, Gillardin J. Modulation of GABAergic synaptic transmission by the non-benzodiazepine anxiolytic etifoxine. Neuropharmacology. 2000; 39:1523–1535. PMID: 10854897.
Article10. Papadopoulos V, Baraldi M, Guilarte TR, Knudsen TB, Lacapère JJ, Lindemann P, et al. Translocator protein (18 kDa): new nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol Sci. 2006; 27:402–409. PMID: 16822554.
Article11. Zeilhofer HU. Etifoxine (Stresam) for chemotherapy-induced pain? Pain. 2009; 147:9–10. PMID: 19822395.
Article12. Kucken AM, Wagner DA, Ward PR, Teissére JA, Boileau AJ, Czajkowski C. Identification of benzodiazepine binding site residues in the gamma 2 subunit of the gamma-aminobutyric acid(A) receptor. Mol Pharmacol. 2000; 57:932–939. PMID: 10779376.13. Sigel E, Steinmann ME. Structure, function, and modulation of GABA(A) receptors. J Biol Chem. 2012; 287:40224–40231. PMID: 23038269.
Article14. Scheller M, Forman SA. The gamma subunit determines whether anesthetic-induced leftward shift is altered by a mutation at alpha1S270 in alpha1beta2gamma2L GABA(A) receptors. Anesthesiology. 2001; 95:123–131. PMID: 11465549.
Article15. Olsen RW, Li GD. GABA(A) receptors as molecular targets of general anesthetics: identification of binding sites provides clues to allosteric modulation. Can J Anaesth. 2011; 58:206–215. PMID: 21194017.
Article16. Rupprecht R, Papadopoulos V, Rammes G, Baghai TC, Fan J, Akula N, et al. Translocator protein (18 kDa) (TSPO) as a therapeutic target for neurological and psychiatric disorders. Nat Rev Drug Discov. 2010; 9:971–988. PMID: 21119734.
Article17. Gunn BG, Brown AR, Lambert JJ, Belelli D. Neurosteroids and GABA(A) Receptor Interactions: A Focus on Stress. Front Neurosci. 2011; 5:131. PMID: 22164129.
Article18. Girard C, Liu S, Cadepond F, Adams D, Lacroix C, Verleye M, et al. Etifoxine improves peripheral nerve regeneration and functional recovery. Proc Natl Acad Sci U S A. 2008; 105:20505–20510. PMID: 19075249.
Article19. Girard C, Liu S, Adams D, Lacroix C, Sinéus M, Boucher C, et al. Axonal regeneration and neuroinflammation: roles for the translocator protein 18 kDa. J Neuroendocrinol. 2012; 24:71–81. PMID: 21951109.
Article20. Zhou X, He X, He B, Zhu Z, Zheng C, Xu J, et al. Etifoxine promotes glial derived neurotrophic factor induced neurite outgrowth in PC12 cells. MOL Med Rep. 2013; 8:75–80. PMID: 23670018.
Article21. Dai T, Zhou X, Li Y, He B, Zhu Z, Zheng C, et al. Etifoxine promotes glia-derived neurite outgrowth in vitro and in vivo. J Reconstr Microsurg. 2014; 30:381–388. PMID: 24956483.
Article22. Zhou X, He B, Zhu Z, He X, Zheng C, Xu J, et al. Etifoxine provides benefits in nerve repair with acellular nerve grafts. Muscle Nerve. 2014; 50:235–243. PMID: 24273088.
Article23. Daugherty DJ, Selvaraj V, Chechneva OV, Liu XB, Pleasure DE, Deng W. A TSPO ligand is protective in a mouse model of multiple sclerosis. EMBO Mol Med. 2013; 5:891–903. PMID: 23681668.
Article24. Aouad M, Charlet A, Rodeau JL, Poisbeau P. Reduction and prevention of vincristine-induced neuropathic pain symptoms by the non-benzodiazepine anxiolytic etifoxine are mediated by 3alpha-reduced neurosteroids. Pain. 2009; 147:54–59. PMID: 19786322.
Article25. Aouad M, Petit-Demoulière N, Goumon Y, Poisbeau P. Etifoxine stimulates allopregnanolone synthesis in the spinal cord to produce analgesia in experimental mononeuropathy. Eur J Pain. 2014; 18:258–268. PMID: 23881562.
Article26. Aouad M, Zell V, Juif PE, Lacaud A, Goumon Y, Darbon P, et al. Etifoxine analgesia in experimental monoarthritis: a combined action that protects spinal inhibition and limits central inflammatory processes. Pain. 2014; 155:403–412. PMID: 24239672.
Article27. Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959; 32:50–55. PMID: 13638508.
Article28. McCracken LM, Zayfert C, Gross RT. The Pain Anxiety Symptoms Scale: development and validation of a scale to measure fear of pain. Pain. 1992; 50:67–73. PMID: 1513605.
Article29. McCracken LM, Dhingra L. A short version of the Pain Anxiety Symptoms Scale (PASS-20): preliminary development and validity. Pain Res Manag. 2002; 7:45–50. PMID: 16231066.
Article30. Micallef J, Soubrouillard C, Guet F, Le Guern ME, Alquier C, Bruguerolle B, et al. A double blind parallel group placebo controlled comparison of sedative and mnesic effects of etifoxine and lorazepam in healthy subjects [corrected]. Fundam Clin Pharmacol. 2001; 15:209–216. PMID: 11468032.
Article31. Nguyen N, Fakra E, Pradel V, Jouve E, Alquier C, Le Guern ME, et al. Efficacy of etifoxine compared to lorazepam monotherapy in the treatment of patients with adjustment disorders with anxiety: a double-blind controlled study in general practice. Hum Psychopharmacol. 2006; 21:139–149. PMID: 16625522.
Article32. Verleye M, Gillardin JM. Effects of etifoxine on stress-induced hyperthermia, freezing behavior and colonic motor activation in rats. Physiol Behav. 2004; 82:891–897. PMID: 15451655.
Article33. Moch C, Rocher F, Lainé P, Lacotte J, Biour M, Gouraud A, et al. Etifoxine-induced acute hepatitis: A case series. Clin Res Hepatol Gastroenterol. 2012; 36:e85–e88. PMID: 22633197.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Study on the Correlation between Pain and Psychological Stress in Endometriosis patients
- What is the gold standard of the dental anxiety scale?
- A Study on Anxiety of the Hospitalized Pregnant Women for Conducting Labor
- The Relationship of Pain, Depression and Anxiety which Patients Recognize on Intravenous Injection: Focus on Pain Relating Factors
- Impacts of Fatigue, Pain, Anxiety, and Depression on the Quality of Life in Patients with Breast Cancer