Korean J Obstet Gynecol.
2011 Jul;54(7):361-368. 10.5468/KJOG.2011.54.7.361.
In vitro suppression of natural killer cell cytolytic activities by prednisolone in patients with history of unexplained recurrent spontaneous abortion and elevated peripheral blood NK cell fraction
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Cheil General Hospital, Women's Healthcare Center, Kwandong University College of Medicine, Seoul, Korea. km1yang@naver.com
- 2Laboratory of Molecular Oncology, Cheil General Hospital, Women's Healthcare Center, Kwandong University College of Medicine, Seoul, Korea.
- 3Laboratory of Reproductive Biology and Infertility, Cheil General Hospital, Women's Healthcare Center, Kwandong University College of Medicine, Seoul, Korea.
- KMID: 2274050
- DOI: http://doi.org/10.5468/KJOG.2011.54.7.361
Abstract
OBJECTIVE
We aimed to evaluate the effect of prednisolone (PDS) on natural killer cell (NK cell) cytolytic activity in vitro.
METHODS
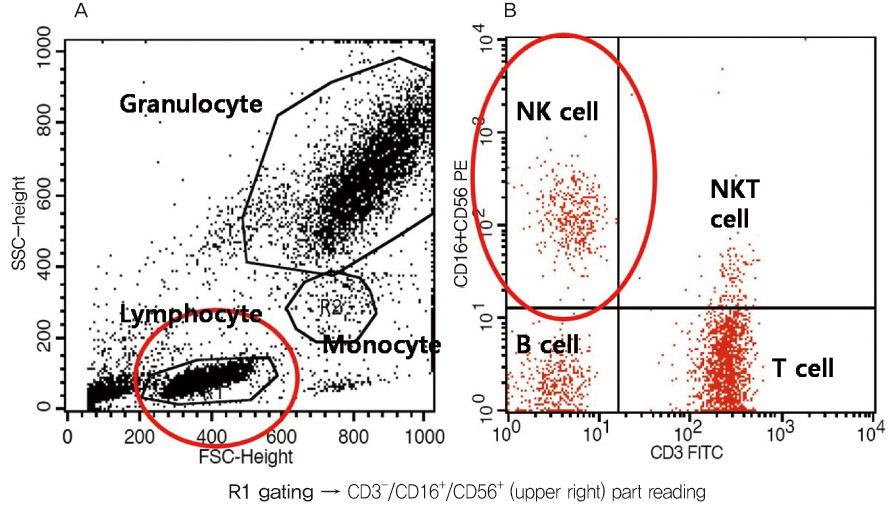
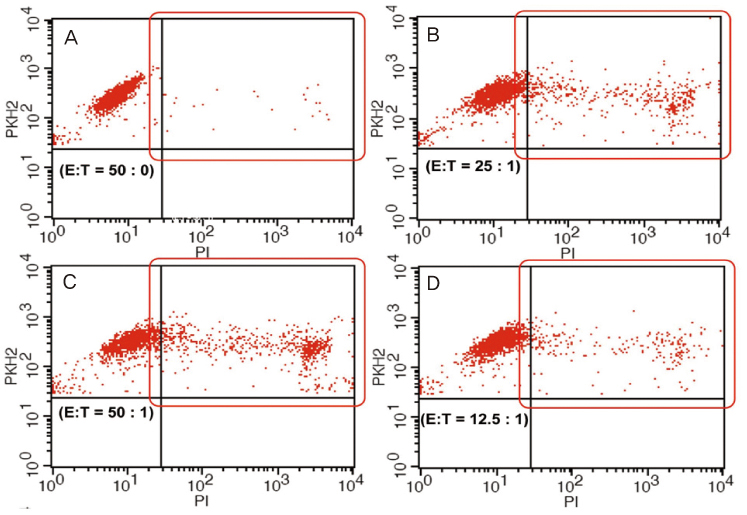
Blood samples from 74 patients with history of unexplained recurrent spontaneous abortion who elevated peripheral blood NK cell fraction were collected prospectively. Peripheral blood monocytes which containing NK cells were isolated and separated to three different tubes which containing target (K562) cells by the 50:1 effector to target (E:T) ratio. PDS or intravenous immunoglobulin (IVIG) was additionally added to 2 tubes for evaluate their suppressive effect. The percentage killing of target cells was recorded numerically by using flow cytometer and the values between groups were statistically analyzed.
RESULTS
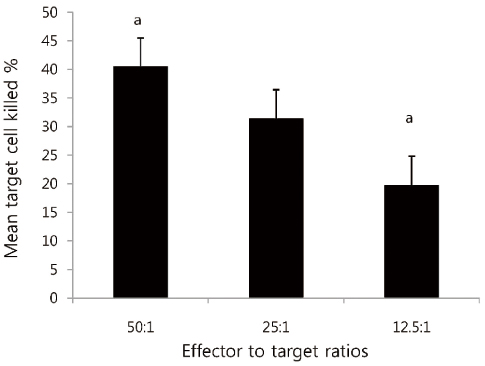
The mean target cell killing percentage was 40.5% in co-culture tube which was not added PDS or IVIG. In culture experiments which was added IVIG, the killing percentage is reduced to 37.7% which showed no significant differences compared to that of co-cultured tube which was not added PDS or IVIG. But, in experiments with added PDS, the killing percent was reduced to 19.5% and the difference was statistically significant (P<0.001) compared to that of co-cultured tube which was not added PDS or IVIG. On comparing the reduction in killing percentage of target cells by PDS and IVIG, statistically significant reduction in the PDS coculture was noted (P<0.005).
CONCLUSION
NK cell cytolytic activity is effectively down-regulated by using PDS in vitro. Moreover, the effect of PDS in down-regulation of NK cell cytolytic activity is seems to superior than that of IVIG. But, large scaled in vivo study is needed.
MeSH Terms
Figure
Reference
-
1. Hannes M, Englert Y, Gotlieb W, Dupont E. Recurrent spontaneous miscarriage. Rev Med Brux. 1992. 13:103–106.2. Stray-Pedersen B, Stray-Pedersen S. Etiologic factors and subsequent reproductive performance in 195 couples with a prior history of habitual abortion. Am J Obstet Gynecol. 1984. 148:140–146.3. Choudhury SR, Knapp LA. Human reproductive failure I: immunological factors. Hum Reprod Update. 2001. 7:113–134.4. Kwak JY, Beaman KD, Gilman-Sachs A, Ruiz JE, Schewitz D, Beer AE. Up-regulated expression of CD56+, CD56+/CD16+, and CD19+ cells in peripheral blood lymphocytes in pregnant women with recurrent pregnancy losses. Am J Reprod Immunol. 1995. 34:93–99.5. Kwak-Kim J, Gilman-Sachs A. Clinical implication of natural killer cells and reproduction. Am J Reprod Immunol. 2008. 59:388–400.6. Beer AE, Kwak JY, Ruiz JE. Immunophenotypic profiles of peripheral blood lymphocytes in women with recurrent pregnancy losses and in infertile women with multiple failed in vitro fertilization cycles. Am J Reprod Immunol. 1996. 35:376–382.7. Coulam CB, Roussev RG. Correlation of NK cell activation and inhibition markers with NK cytoxicity among women experiencing immunologic implantation failure after in vitro fertilization and embryo transfer. J Assist Reprod Genet. 2003. 20:58–62.8. Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001. 22:633–640.9. Cooper MA, Fehniger TA, Turner SC, Chen KS, Ghaheri BA, Ghayur T, et al. Human natural killer cells: a unique innate immunoregulatory role for the CD56(bright) subset. Blood. 2001. 97:3146–3151.10. Wegmann TG, Lin H, Guilbert L, Mosmann TR. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol Today. 1993. 14:353–356.11. Higuchi K, Aoki K, Kimbara T, Hosoi N, Yamamoto T, Okada H. Suppression of natural killer cell activity by monocytes following immunotherapy for recurrent spontaneous aborters. Am J Reprod Immunol. 1995. 33:221–227.12. Yamada H, Morikawa M, Kato EH, Shimada S, Kobashi G, Minakami H. Pre-conceptional natural killer cell activity and percentage as predictors of biochemical pregnancy and spontaneous abortion with normal chromosome karyotype. Am J Reprod Immunol. 2003. 50:351–354.13. Ronda N, Hurez V, Kazatchkine MD. Intravenous immunoglobulin therapy of autoimmune and systemic inflammatory diseases. Vox Sang. 1993. 64:65–72.14. Prasad NK, Papoff G, Zeuner A, Bonnin E, Kazatchkine MD, Ruberti G, et al. Therapeutic preparations of normal polyspecific IgG (IVIg) induce apoptosis in human lymphocytes and monocytes: a novel mechanism of action of IVIg involving the Fas apoptotic pathway. J Immunol. 1998. 161:3781–3790.15. Scott JR, Branch DW, Kochenour NK, Ward K. Intravenous immunoglobulin treatment of pregnant patients with recurrent pregnancy loss caused by antiphospholipid antibodies and Rh immunization. Am J Obstet Gynecol. 1988. 159:1055–1056.16. Kwak JY, Quilty EA, Gilman-Sachs A, Beaman KD, Beer AE. Intravenous immunoglobulin infusion therapy in women with recurrent spontaneous abortions of immune etiologies. J Reprod Immunol. 1995. 28:175–188.17. Coulam CB, Krysa L, Stern JJ, Bustillo M. Intravenous immunoglobulin for treatment of recurrent pregnancy loss. Am J Reprod Immunol. 1995. 34:333–337.18. Christiansen OB, Mathiesen O, Husth M, Rasmussen KL, Ingerslev HJ, Lauritsen JG, et al. Placebo-controlled trial of treatment of unexplained secondary recurrent spontaneous abortions and recurrent late spontaneous abortions with i.v. immunoglobulin. Hum Reprod. 1995. 10:2690–2695.19. The German RSA/IVIG Group. Intravenous immunoglobulin in the prevention of recurrent miscarriage. Br J Obstet Gynaecol. 1994. 101:1072–1077.20. Stephenson MD, Dreher K, Houlihan E, Wu V. Prevention of unexplained recurrent spontaneous abortion using intravenous immunoglobulin: a prospective, randomized, double-blinded, placebo-controlled trial. Am J Reprod Immunol. 1998. 39:82–88.21. Kim MY, Kim HS, Cha SH, Koong MK, Song HJ, Song IO, et al. A change of peripheral CD56+natural killer cell fraction in recurrent pregnancy loss patients who successfully treated with intravenous immunoglobulin in previous pregnancy. Korean J Obstet Gynecol. 2004. 47:1926–1930.22. Cha SH, Park CW, Kim HS, Cho DH, Kim JY, Kang IS, et al. Effectiveness of intravenous immunoglobulin therapy in women with recurrent spontaneous abortions and elevated pre-conceptional peripheral blood CD56+ natural killer cell percentage. Korean J Fertil Steril. 2005. 32:165–170.23. Forges T, Monnier-Barbarino P, Guillet-May F, Faure GC, Béné MC. Corticosteroids in patients with antiovarian antibodies undergoing in vitro fertilization: a prospective pilot study. Eur J Clin Pharmacol. 2006. 62:699–705.24. Taniguchi F. Results of prednisolone given to improve the outcome of in vitro fertilization-embryo transfer in women with antinuclear antibodies. J Reprod Med. 2005. 50:383–388.25. Quenby S, Kalumbi C, Bates M, Farquharson R, Vince G. Prednisolone reduces preconceptual endometrial natural killer cells in women with recurrent miscarriage. Fertil Steril. 2005. 84:980–984.26. Hasegawa I, Yamanoto Y, Suzuki M, Murakawa H, Kurabayashi T, Takakuwa K, et al. Prednisolone plus low-dose aspirin improves the implantation rate in women with autoimmune conditions who are undergoing in vitro fertilization. Fertil Steril. 1998. 70:1044–1048.27. Mottla GL, Smotrich DB, Gindoff PR, Stillman RJ. Increasing clinical pregnancy rates after IVF/ET. Can immunosuppression help? J Reprod Med. 1996. 41:889–891.28. Ando T, Suganuma N, Furuhashi M, Asada Y, Kondo I, Tomoda Y. Successful glucocorticoid treatment for patients with abnormal autoimmunity on in vitro fertilization and embryo transfer. J Assist Reprod Genet. 1996. 13:776–781.29. Polak de Fried E, Blanco L, Lancuba S, Asch RH. Improvement of clinical pregnancy rate and implantation rate of in-vitro fertilization-embryo transfer patients by using methylprednisone. Hum Reprod. 1993. 8:393–395.30. Henderson TA, Saunders PT, Moffett-King A, Groome NP, Critchley HO. Steroid receptor expression in uterine natural killer cells. J Clin Endocrinol Metab. 2003. 88:440–449.31. Chiossone L, Vitale C, Cottalasso F, Moretti S, Azzarone B, Moretta L, et al. Molecular analysis of the methylprednisolone-mediated inhibition of NK-cell function: evidence for different susceptibility of IL-2- versus IL-15-activated NK cells. Blood. 2007. 109:3767–3775.32. Thum MY, Bhaskaran S, Abdalla HI, Ford B, Sumar N, Bansal A. Prednisolone suppresses NK cell cytotoxicity in vitro in women with a history of infertility and elevated NK cell cytotoxicity. Am J Reprod Immunol. 2008. 59:259–265.33. Daya S. Copeland LJ, Jarrell JF, McGregor JA, editors. Habitual abortion. Text book of gynecology. 1993. Philadelphia (PA): WB Saunders Company;204–230.34. Daya S. Immunotherapy for unexplained recurrent spontaneous abortion. Infertil Reprod Med Clin North Am. 1997. 8:65–77.35. Aoki K, Kajiura S, Matsumoto Y, Ogasawara M, Okada S, Yagami Y, et al. Preconceptional natural-killer-cell activity as a predictor of miscarriage. Lancet. 1995. 345:1340–1342.36. Emmer PM, Veerhoek M, Nelen WL, Steegers EA, Joosten I. Natural killer cell reactivity and HLA-G in recurrent spontaneous abortion. Transplant Proc. 1999. 31:1838–1840.37. Lanier LL, Le AM, Ding A, Evans EL, Krensky AM, Clayberger C, et al. Expression of Leu-19 (NKH-1) antigen on IL 2-dependent cytotoxic and non-cytotoxic T cell lines. J Immunol. 1987. 138:2019–2023.38. Yokoyama M, Sano M, Sonoda K, Nozaki M, Nakamura G, Nakano H. Cytotoxic cells directed against placental cells detected in human habitual abortions by an in vitro terminal labeling assay. Am J Reprod Immunol. 1994. 31:197–204.39. Ruiz JE, Kwak JY, Baum L, Gilman-Sachs A, Beaman KD, Kim YB, et al. Intravenous immunoglobulin inhibits natural killer cell activity in vivo in women with recurrent spontaneous abortion. Am J Reprod Immunol. 1996. 35:370–375.40. Daya S, Gunby J, Porter F, Scott J, Clark DA. Critical analysis of intravenous immunoglobulin therapy for recurrent miscarriage. Hum Reprod Update. 1999. 5:475–482.41. Dwyer JM. Manipulating the immune system with immune globulin. N Engl J Med. 1992. 326:107–116.42. Xu B, Makris A, Thornton C, Hennessy A. Glucocorticoids inhibit placental cytokines from cultured normal and preeclamptic placental explants. Placenta. 2005. 26:654–660.43. Steer JH, Vuong Q, Joyce DA. Suppression of human monocyte tumour necrosis factor-alpha release by glucocorticoid therapy: relationship to systemic monocytopaenia and cortisol suppression. Br J Clin Pharmacol. 1997. 43:383–389.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Changes of natural killer cell number and cytolytic activity during first trimester of pregnancy in recurrent spontaneous abortion patients and fertile control
- The Preconceptional Level of Peripheral Natural Killer Cells which was Expected to Bring Successful Treatment Outcome using Low-dose Intravenous Gamma Immunoglobulin (IVIg) Infusion in Patients with Recurrent Spontaneous Abortion
- Resistance of uterine radial artery blood flow is positively correlated with peripheral blood NK cell fraction in patients with unexplained recurrent spontaneous abortion
- Elevated natural killer cell levels and autoimmunity synergistically decrease uterine blood flow during early pregnancy
- A Change of Peripheral CD56+Natural Killer Cell Fraction in Recurrent Pregnancy Loss Patients who Successfully Treated with Intravenous Immunoglobulin in Previous Pregnancy