Obstet Gynecol Sci.
2014 May;57(3):208-215. 10.5468/ogs.2014.57.3.208.
Elevated natural killer cell levels and autoimmunity synergistically decrease uterine blood flow during early pregnancy
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Cheil General Hospital and Women's Healthcare Center, Kwandong University College of Medicine, Seoul, Korea. km1yang@naver.com
- KMID: 1704427
- DOI: http://doi.org/10.5468/ogs.2014.57.3.208
Abstract
OBJECTIVE
To investigate whether natural killer (NK) cell and autoimmune antibody acts synergistically, by the action of autoantibodies to increase NK cell number and cytotoxicity, to decrease uterine blood flow during early pregnancy in pregnant women with a history of recurrent spontaneous abortion (RSA).
METHODS
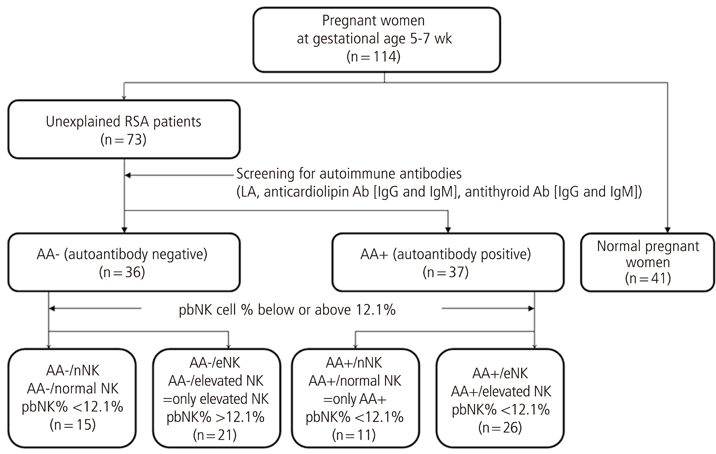
Seventy-five pregnant women (between 5 and 7 weeks gestation) with a history of unexplained RSA were included in the study group. Forty-one pregnant women without a history of RSA were included as controls. All women with a history of RSA were tested for autoantibodies and number of peripheral blood natural killer (pbNK) cell by flow cytometry. Study populations were stratified into four groups by existence of autoantibody and degree of increase of pbNK cells. The uterine radial artery resistance index (RI) was measured by color-pulsed Doppler transvaginal ultrasound.
RESULTS
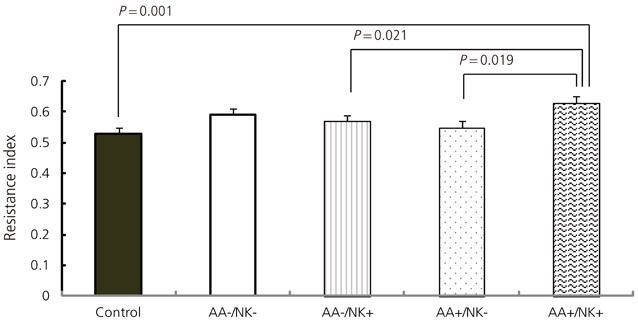
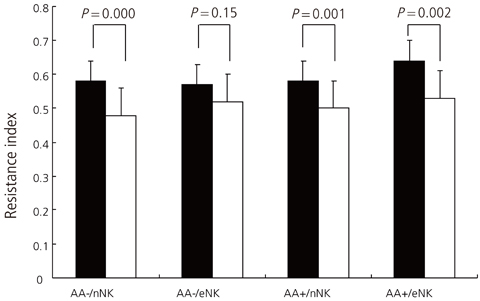
The mean RI of the autoimmune antibody-positive (AA+) group (0.63+/-0.09) was significantly higher than that of the normal control group (0.53+/-0.10, P=0.001). The mean RI of the AA+/only-NK elevated (eNK) group (0.63+/-0.09) was significantly higher than those of the only-AA+ group (0.55+/-0.07, P=0.019) and the only-eNK group (0.57+/-0.07, P=0.021).
CONCLUSION
Concurrent elevation in NK cells and autoimmunity results in decreased uterine blood flow during early pregnancy. However, the majority of cases of RSA remain unexplained and larger scale studies are needed to confirm our conclusion and to develop diagnostic and therapeutic plans for women with a history of RSA.
MeSH Terms
Figure
Reference
-
1. Stephenson MD. Frequency of factors associated with habitual abortion in 197 couples. Fertil Steril. 1996; 66:24–29.2. Matthiesen L, Kalkunte S, Sharma S. Multiple pregnancy failures: an immunological paradigm. Am J Reprod Immunol. 2012; 67:334–340.3. Tulppala M, Palosuo T, Ramsay T, Miettinen A, Salonen R, Ylikorkala O. A prospective study of 63 couples with a history of recurrent spontaneous abortion: contributing factors and outcome of subsequent pregnancies. Hum Reprod. 1993; 8:764–770.4. Plouffe L Jr, White EW, Tho SP, Sweet CS, Layman LC, Whitman GF, et al. Etiologic factors of recurrent abortion and subsequent reproductive performance of couples: have we made any progress in the past 10 years? Am J Obstet Gynecol. 1992; 167:313–320.5. Hanna J, Goldman-Wohl D, Hamani Y, Avraham I, Greenfield C, Natanson-Yaron S, et al. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med. 2006; 12:1065–1074.6. Perricone R, Di Muzio G, Perricone C, Giacomelli R, De Nardo D, Fontana L, et al. High levels of peripheral blood NK cells in women suffering from recurrent spontaneous abortion are reverted from high-dose intravenous immunoglobulins. Am J Reprod Immunol. 2006; 55:232–239.7. Perricone C, de Carolis C, Perricone R. Pregnancy and autoimmunity: a common problem. Best Pract Res Clin Rheumatol. 2012; 26:47–60.8. Kwak-Kim J, Park JC, Ahn HK, Kim JW, Gilman-Sachs A. Immunological modes of pregnancy loss. Am J Reprod Immunol. 2010; 63:611–623.9. Pratt DE, Kaberlein G, Dudkiewicz A, Karande V, Gleicher N. The association of antithyroid antibodies in euthyroid nonpregnant women with recurrent first trimester abortions in the next pregnancy. Fertil Steril. 1993; 60:1001–1005.10. Bussen S, Steck T. Thyroid autoantibodies in euthyroid non-pregnant women with recurrent spontaneous abortions. Hum Reprod. 1995; 10:2938–2940.11. Sladkevicius P, Valentin L, Marsal K. Blood flow velocity in the uterine and ovarian arteries during the normal menstrual cycle. Ultrasound Obstet Gynecol. 1993; 3:199–208.12. Habara T, Nakatsuka M, Konishi H, Asagiri K, Noguchi S, Kudo T. Elevated blood flow resistance in uterine arteries of women with unexplained recurrent pregnancy loss. Hum Reprod. 2002; 17:190–194.13. Ferreira AM, Pires CR, Moron AF, Araujo Junior E, Traina E, Mattar R. Doppler assessment of uterine blood flow in recurrent pregnancy loss. Int J Gynaecol Obstet. 2007; 98:115–119.14. Tamura H, Miwa I, Taniguchi K, Maekawa R, Asada H, Taketani T, et al. Different changes in resistance index between uterine artery and uterine radial artery during early pregnancy. Hum Reprod. 2008; 23:285–289.15. Choi JY, Hwang SJ, Han AR, Yoo JH, Park DW, Park CW, et al. Increased peripheral NK cell fraction and their cytolytic activity in patients with history of recurrent spontaneous abortion. Korean J Reprod Med. 2010; 37:115–124.16. Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006; 4:295–306.17. Kwak JY, Beaman KD, Gilman-Sachs A, Ruiz JE, Schewitz D, Beer AE. Up-regulated expression of CD56+, CD56+/CD16+, and CD19+ cells in peripheral blood lymphocytes in pregnant women with recurrent pregnancy losses. Am J Reprod Immunol. 1995; 34:93–99.18. Kim NY, Cho HJ, Kim HY, Yang KM, Ahn HK, Thornton S, et al. Thyroid autoimmunity and its association with cellular and humoral immunity in women with reproductive failures. Am J Reprod Immunol. 2011; 65:78–87.19. Steer CV, Tan SL, Mason BA, Campbell S. Midluteal-phase vaginal color Doppler assessment of uterine artery impedance in a subfertile population. Fertil Steril. 1994; 61:53–58.20. Goswamy RK, Williams G, Steptoe PC. Decreased uterine perfusion: a cause of infertility. Hum Reprod. 1988; 3:955–959.21. Hui C, Lili M, Libin C, Rui Z, Fang G, Ling G, et al. Changes in coagulation and hemodynamics during pregnancy: a prospective longitudinal study of 58 cases. Arch Gynecol Obstet. 2012; 285:1231–1236.22. El-mashad AI, Mohamed MA, Farag MA, Ahmad MK, Ismail Y. Role of uterine artery Doppler velocimetry indices and plasma adrenomedullin level in women with unexplained recurrent pregnancy loss. J Obstet Gynaecol Res. 2011; 37:51–57.23. Frates MC, Doubilet PM, Brown DL, Benson CB, DiSalvo DN, Laing FC, et al. Role of Doppler ultrasonography in the prediction of pregnancy outcome in women with recurrent spontaneous abortion. J Ultrasound Med. 1996; 15:557–562.24. Ng EH, Chan CC, Tang OS, Yeung WS, Ho PC. Endometrial and subendometrial vascularity is higher in pregnant patients with livebirth following ART than in those who suffer a miscarriage. Hum Reprod. 2007; 22:1134–1141.25. Quenby S, Bates M, Doig T, Brewster J, Lewis-Jones DI, Johnson PM, et al. Pre-implantation endometrial leukocytes in women with recurrent miscarriage. Hum Reprod. 1999; 14:2386–2391.26. Gris JC. LMWH have no place in recurrent pregnancy loss: debate-against the motion. Thromb Res. 2011; 127:Suppl 3. S110–S112.27. Venkat-Raman N, Backos M, Teoh TG, Lo WT, Regan L. Uterine artery Doppler in predicting pregnancy outcome in women with antiphospholipid syndrome. Obstet Gynecol. 2001; 98:235–242.28. Dolitzky M, Inbal A, Segal Y, Weiss A, Brenner B, Carp H. A randomized study of thromboprophylaxis in women with unexplained consecutive recurrent miscarriages. Fertil Steril. 2006; 86:362–366.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Resistance of uterine radial artery blood flow is positively correlated with peripheral blood NK cell fraction in patients with unexplained recurrent spontaneous abortion
- Changes of natural killer cell number and cytolytic activity during first trimester of pregnancy in recurrent spontaneous abortion patients and fertile control
- Pregnancy outcomes following the administration of high doses of dexamethasone in early pregnancy
- Deficiencies of Circulating Mucosal-associated Invariant T Cells and Natural Killer T Cells in Patients with Multiple Trauma
- Effectiveness of Intravenous Immunoglobulin Therapy in Women with Recurrent Spontaneous Abortions and Elevated Pre-conceptional Peripheral Blood CD56+ Natural Killer Cell Percentage