Asian Oncol Nurs.
2014 Jun;14(2):93-99. 10.5388/aon.2014.14.2.93.
Comparison of High Dose Methotrexate Administration Between the Inpatient and Outpatient Setting in Children with Acute Lymphoblastic Leukemia
- Affiliations
-
- 1BMT Center, Seoul St. Mary's Hospital, The Catholic University of Korea, Seoul, Korea. bm.coor@cmcnu.or.kr
- KMID: 2269463
- DOI: http://doi.org/10.5388/aon.2014.14.2.93
Abstract
- PURPOSE
Methotrexate (MTX) is one of the most widely used anticancer agents, with indications and established protocols in a range of childhood and adult cancers. High dose MTX (HD-MTX) requires aggressive care to prevent toxicity. Limited inpatient conditions are forcing major changes in health care delivery patterns and decisions. We conducted a retrospective study to describe the safety, feasibility and cost-effectiveness of HD-MTX administration in the outpatient setting.
METHODS
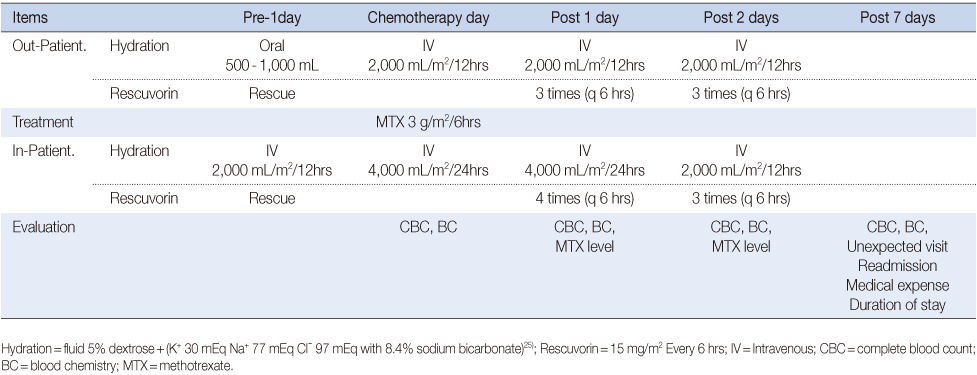
Patients with acute lymphoblastic leukemia who underwent HD-MTX (3 g/m2) administration in either the inpatient (N=70) or outpatient setting (N=70) from January to July 2012 were included. In the outpatient setting, HD-MTX was administered intravenously (IV) over 6 hours and included hydration with sodium bicarbonate (2000 ml/m2/for 12 hours). Daily visits to the outpatient setting followed. Leucovorin was given 24 hours after MTX at a standard dose (15 mg/m2 IV bolus) every 6 hours. We compared the serum drug levels of MTX, hematologic and renal toxicity, hepatotoxicity, frequency of subsequent unscheduled outpatient visits and readmission episodes, medical expenses and duration of hospital stay between the two groups.
RESULTS
HD-MTX administrations were successfully completed in both groups. No significant differences were found between the two groups for the parameters studied. Patients who received HD-MTX in the inpatient setting had 2.37 times and 2.24 times greater medical expenses and duration of hospital stay respectively than outpatient recipients.
CONCLUSION
This study suggests that HD-MTX administration done with aggressive monitoring in the outpatient setting is safe and efficient, without a greater incidence of major toxicities.
MeSH Terms
Figure
Cited by 2 articles
-
Job Analysis Based on Working Hours and Activities of Oncology Advanced Practice Nurses
Hye Jin Joh, Jee Hyun Lee, Sun Hee Choi, Hye Kyung Kim, Kwang Sung Kim
Asian Oncol Nurs. 2015;15(1):43-50. doi: 10.5388/aon.2015.15.1.43.Physical, Psychological and Social Symptoms, Activity and Education of Children and Adolescents with Acute Lymphoblastic Leukemia Receiving Maintenance Chemotherapy
Hee Sung Yoon, Kwang Sung Kim, Sun Hee Choi, So Eun Choi, Kyoung A Kim, Kyoung Eon Kim
Asian Oncol Nurs. 2016;16(4):169-175. doi: 10.5388/aon.2016.16.4.169.
Reference
-
1. Statistics Korea. http://kostat.go.kr. Accessed September 1, 2013.2. Pui CH, Carroll WL, Meshinchi S, Arceci RJ. Biology, risk stratification, and therapy of pediatric acute leukemias: an update. J Clin Oncol. 2011; 29:551–565.
Article3. Pui CH, Relling MV, Downing JR. Acute lymphoblastic leukemia. N Engl J Med. 2004; 350:1535–1548.
Article4. Moricke A, Zimmermann M, Reiter A, Henze G, Schrauder A, Gadner H, et al. Long-term results of five consecutive trials in childhood acute lymphoblastic leukemia performed by the ALL-BFM study group from 1981 to 2000. Leukemia. 2010; 24:265–284.
Article5. Seibel NL. Treatment of acute lymphoblastic leukemia in children and adolescents peaks and pitfalls. Hematology Am Soc Hematol Educ Program. 2008; 374–380.
Article6. Mahadeo KM, Santizo R, Baker L, Curry JO, Gorlick R, Levy AS. Ambulatory high-dose methotrexate administration among pediatric osteosarcoma patients in an urban, underserved setting is feasible, safe, and cost-effective. Pediatr Blood Cancer. 2010; 55:1296–1299.
Article7. Pui CH, Robison LL, Look AT. Acute lymphoblastic leukaemia. Lancet. 2008; 371:1030–1043.
Article8. Miller DR. A tribute to Sidney Farber--the father of modern chemotherapy. Br J Haematol. 2006; 134:20–26.
Article9. Allergra CJ, Grem JL. Antimetabolites. In : Devita VT, Hellman S, Rosenberg SA, editors. Cancer : Principles and practice of oncology. Philadelphia: Lippincott;1997. p. 432–465.10. Evans WE, Schentag JJ, Jusko WJ. Applied pharmacokinetics. Vancouver, WA: Applied Therapeutics;1992. p. 29–42.11. Wilke WS, Mackenzie AH. Methotrexate therapy in rheumatoid arthritis Current status. Drugs. 1986; 32:103–113.
Article12. Blum R, Seymour JF, Toner G. Significant impairment of high-dose methotrexate clearance following vancomycin administration in the absence of overt renal impairment. Ann Oncol. 2002; 13:327–330.
Article13. Schmiegelow K. Advances in individual prediction of methotrexate toxicity: a review. Br J Haematol. 2009; 146:489–503.
Article14. Aquerreta I, Aldaz A, Giráldez J, Sierrasesumaga L. Pharmacodynamics of high-dose methotrexate in pediatric patients. Ann Pharmacother. 2002; 36:1344–1350.
Article15. Zelcer S, Kellick M, Wexler LH, Gorlick R, Meyers PA. The Memorial Sloan Kettering Cancer Center experience with outpatient administration of high dose methotrexate with leucovorin rescue. Pediatr Blood Cancer. 2008; 50:1176–1180.
Article16. Wong DL, Hockenberry MJ, Wilson D. Wong's nursing care of infants and children. 9th ed. St. Louis, Mo: Mosby/Elsevier;2009. p. 1253–1263.17. Jolivet J, Cowan KH, Curt GA, Clendeninn NJ, Chabner BA. The pharmacology and clinical use of methotrexate. N Engl J Med. 1983; 309:1094–1104.
Article18. US Department of Health and Human Service. Common terminology criteria for adverse events (CTCAE) Version 4.0. 2009.19. Nancy E, Joy E. The pediatric chemotherapy and biotherapy curriculum. 3rd ed. Glenview, IL: Association of Pediatric Hematology/Oncology Nurses;2011. p. 106.20. American Pharmacists Association. Drug information handbook with international trade name index. Lexicomp;2012.21. Lanzkowsky P. Manual of pediatric hematology and oncology. 5th ed. Amsterdam, Boston: Academin Press;2010.22. Rahiem Ahmed YAA, Hasan Y. Prevention and management of high dose methotrexate toxicity. J Cancer Sci Ther. 2013; 5:106–112.
Article23. Martha E, Janet D, Shirley E. Oncology nursing. 5th ed. St. Louis, Mo: Mosby/Elsevier;2007.24. Mir O, Ropert S, Babinet A, Alexandre J, Larousserie F, Durand JP, et al. Hyper-alkalinization without hyper-hydration for the prevention of high-dose methotrexate acute nephrotoxicity in patients with osteosarcoma. Cancer Chemother Pharmacol. 2010; 66:1059–1063.
Article25. 2013 CMC medical index. The catholic medical center;2013. p. 881.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Low Dose Methotrexate induced Bullous Acral Erythema in a Child with Acute Lymphoblastic Leukemia
- A Case of Paraplegia Associated with Intrathecal Methotrexate : A case report
- A case of aseptic meningitis following intrathecal administration of methotrexate
- Acute Management of Intrathecal Methotrexate Overdose in a Patient with Acute Lymphocytic Leukemia
- Paraplegia Due to Spinal Hematoma in a Patient with Acute Lymphocytic Leukemia: A case report