Korean J Nutr.
2011 Aug;44(4):284-291. 10.4163/kjn.2011.44.4.284.
Effects of Water Extracts of Red Pepper Seeds Powder on Antioxidative Enzyme Activities and Oxidative Damage in Rats Fed High-Fat and High-Cholesterol Diets
- Affiliations
-
- 1Department of Food Science, International University of Korea, Jinju 660-759, Korea.
- 2Korea Food Research Institute, Seongnam 463-746, Korea.
- 3Department of Food Science and Nutrition, International University of Korea, Jinju 660-759, Korea. jhappychoi@hanmail.net
- KMID: 2268544
- DOI: http://doi.org/10.4163/kjn.2011.44.4.284
Abstract
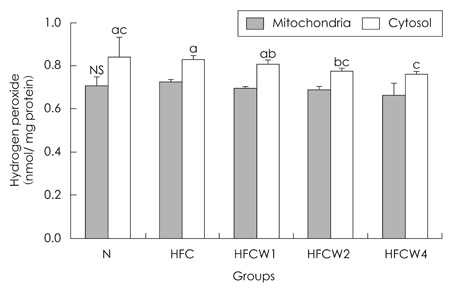
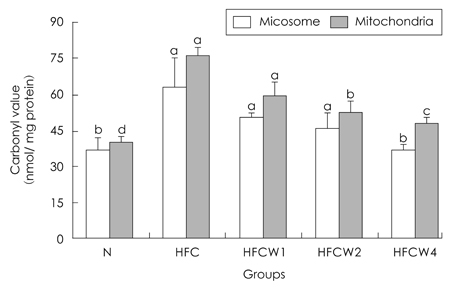
- The purpose of the present study was to examine the effects of water extracts from red pepper seeds powder on antioxidative enzyme activities and oxidative damage in groups of rrats fed high-fat and high-cholesterol diets group (HFC). The Rrats were divided into the following five experimental groups which are : composed ofa normal diet group, a high fat.high cholesterol diet group, and a high fat.high cholesterol diet group supplemented with different amounts contents (1%, 2% and 4%) of red pepper seeds powder water extracts supplemented groups (HFCW1, HFCW2 and HFCW4, respectively). Body weight gains and food intake were lower ofin the red pepper seed water extracts groups were lower than those inof the HFC group. Hepartic xanthine oxidase (XOD) activity was decreased in the HFCW2 and HFCW4 groups compared to the HFC group. Hepartic glutathione peroxidase (GSH-px) activitiyactivity was increased in the HFCW4 group compared to the HFC group. Hepatic superoxide radicals within the mitochondria and microsomes of cells were significantly reduced in the HFCW2 and HFCW4 groups compared to the HFC group. Hepartic hydrogen peroxide in the cytosol was significantly reduced in the HFCW3 and HFCW4 groups compared to the HFC group. Hepatic carbonyl values in the microsomes and mitochondria were significantly reduced in the HFCW4 group compared to the HFC group. Hepartic thiobarbituric acid reaction substance (TBARS) activity was decreased in the HFCW2 group compared to the HFC group. These results suggest that water extracts of red pepper seeds powder may reduce oxidative damage by activation of antioxidative defense systems in rats fed high fat.high cholesterol diets.
Keyword
MeSH Terms
Figure
Reference
-
1. Ko SC, Kang SM, Ahn G, Yang HP, Kim KN, Jeon YJ. Antioxidant activity of enzymatic extracts from sargassum coreanum. J Korean Soc Food Sci Nutr. 2010. 39(4):494–499.
Article2. Jang JR, Hwang SY, Lim SY. Effects of extracts from dried Yam on antioxidant and growth of human cancer cell lines. J Life Sci. 2010. 20(9):1365–1372.
Article3. Yokozawa T, Nakagawa T, Kitani K. Antioxidative activity of green tea polyphenol in cholesterol-fed rats. J Agric Food Chem. 2002. 50(12):3549–3552.
Article4. Bok SH, Park SY, Park YB, Lee MK, Jeon SM, Jeong TS, Choi MS. Quercetin dihydrate and gallate supplements lower plasma and hepatic lipids and change activities of hepatic antioxidant enzymes in high cholesterol-fed rats. Int J Vitam Nutr Res. 2002. 72(3):161–169.
Article5. Balkan J, Kanbağli O, Hatipoğlu A, Kücük M, Cevikbuş U, Aykaç-Toker G, Uysal M. Improving effect of dietary taurine supplementation on the oxidative stress and lipid levels in the plasma, liver and aorta of rabbits fed on a high-cholesterol diet. Biosci Biotechnol Biochem. 2002. 66(8):1755–1758.
Article6. Rhee SJ, Ahn JM, Ku KH, Choi JH. Effects of radish leaves powder on hepatic antioxidative system in rats fed high-cholesterol diet. J Korean Soc Food Sci Nutr. 2005. 34(8):1157–1163.
Article7. Lee YM, Bae JH, Jung HY, Kim JH, Park DS. Antioxidant activity in water and methanol extracts from korean edible wild plants. J Korean Soc Food Sci Nutr. 2011. 40(1):29–36.
Article8. Liu RH. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am J Clin Nutr. 2003. 78:3 Suppl. 517S–520S.
Article9. Yoon J, Jun JJ, Lim SC, Lee KH, Kim HT, Jeong HS, Lee J. Changes in selected components and antioxidant and antiproliferative activity of peppers depending on cultivation. J Korean Soc Food Sci Nutr. 2010. 39(5):731–736.
Article10. Sim KH, Han YS. The Antimutagenic and Antioxidant effects of red pepper seed and red pepper pericarp (Capsicum annuum L.). J Food Sci Nutr. 2007. 12(4):273–278.
Article11. Choi SM, Jeon YS, Jung KO, Park KY. Antimutagenic effects of different kinds and parts of red pepper/powder on the N-methyl-N'-nitro-N-nitrosoguanidine (MNNG)-induced mutagenicities. J Korean Assoc Cancer Prev. 2001. 6(2):108–115.12. Sim KH, Kim SI, Cho YK, Cho YS, Han YS. The effects of red pepper seed on Kimchi quality during fermentation. Food Qual Cult. 2007. 1(1):27–33.13. Ku KH, Choi EJ, Park JB. Chemical component analysis of red pepper (Capsicum annuum L.) seeds with various cultivars. J Korean Soc Food Sci Nutr. 2008. 37(8):1084–1089.
Article14. Stirpe F, Corte ED. The regulation of rat liver xanthine oxidase. Conversion in vitro of the enzyme activity from dehydrogenase (type D) to oxidase (type O). J Biol Chem. 1969. 244(14):3855–3386.15. MarKlund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974. 47(3):469–474.
Article16. Lawrence RA, Burk RF. Glutathione peroxidase activity in selenium-deficient rat liver. Biochem Biophys Res Commun. 1976. 71(4):952–958.
Article17. Aebi H, Wyss SR, Scherz B, Skvaril F. Heterogeneity of erythrocyte catalase II. Isolation and characterization of normal and variant erythrocyte catalase and their subunits. Eur J Biochem. 1974. 48(1):137–145.
Article18. Gay C, Gebicki JM. A critical evaluation of the effect of sorbitol on the ferric-xylenol orange hydroperoxide assay. Anal Biochem. 2000. 284(2):217–220.
Article19. Azzi A, Montecucco C, Richter C. The use of acetylated ferricytochrome c for the detection of superoxide radicals produced in biological membranes. Biochem Biophys Res Commun. 1975. 65(2):597–603.
Article20. Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG, Ahn BW, Shaltiel S, Stadtman ER. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990. 186:464–478.21. Satoh k. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin Chim Acta. 1978. 90(1):37–43.
Article22. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biochem. 1951. 193(1):265–275.
Article23. Steel RGD, Torrie JH. Principles and procedures of statistics. 1990. New York, USA: McGraw-Hill.24. Zhang XH, Choi SK, Seo JS. Effect of dietary grape pomace on lipid oxidation and related enzyme activities in rats fed high fat diet. Korean J Nutr. 2009. 42(5):415–422.
Article25. Suh SH, Lee HR, Rhee SJ, Choi SW, Cho SH. Effects of peonia seed extracts and resveratrol on lipid metabolism in rats fed high cholesterol diets. J Korean Soc Food Sci Nutr. 2003. 32(7):1102–1107.
Article26. Duke EJ, Joyce P, Ryan JP. Characterization of alternative molecular forms of xanthine oxidase in the mouse. Biochem J. 1973. 131(2):187–190.
Article27. Ham YK, Kim SW. Protective effects of plant extracts of plant extract on the hepatocytes of rat treated with carbon tetrachloride. J Korean Soc Food Sci Nutr. 2004. 33(8):1246–1251.28. Urano S, Hoshi-Hashizume M, Tochigi N, Matsuo M, Shiraki M, Ito H. Vitamin E and the susceptibility of erythrocytes and reconstituted liposome to oxidative stress in aged diabetics. Lipids. 1991. 26(1):58–61.
Article29. Kim HJ. Antioxidant and physialogical activities of capsicum annuum extract [dissertation]. 2010. Seoul: Korea University.30. Song WY, Ku KH, Choi JH. Effect of ethanol extracts from red pepper seeds on antioxidative defense system and oxidative stress in rats fed high-fat, high-cholesterol diet. Nutr Res Pract. 2010. 4(1):11–15.
Article31. Park YS. Antioxidative activities and contents of polyphenolic compound of medicinal herb extracts. J East Asian Soc Diet Life. 2002. 12(1):23–31.32. Kim SM, Jung YJ, Pan CH, Um BH. Antioxidant activity of methanol extracts from the genus lespedeza. J Korean Soc Food Sci Nutr. 2010. 39(5):769–775.
Article33. Ku KH, Choi EJ, Park WS. Functional activity of water and ethanol extracts from red pepper (Capsicum annuum L.) seeds. J Korean Soc Food Sci Nutr. 2008. 37(10):1357–1136.
Article34. Lee J, Lee SR. Analysis of phenolic substances content in Korean plant foods. Korean J Food Sci Technol. 1994. 26(3):310–316.35. Moon SH, Lee MK. Inhibitory effects of xanthine oxidase by boiled water extract and tannin from persimmon leaves. Korean J Food Nutr. 2001. 11(3):354–357.36. Yeo SG, Park YB, Kim IS, Kim SB, Park YH. Inhibitory effects of xanthine oxidase by tea extract from green tee, oolong tea, and black tea. J Korean Soc Food Sci Nutr. 1995. 24:154–159.37. Ki HS, Han YS. Antioxidant activities of red pepper (Capsicum annuum) pericarp and seed extracts. Int J Food Sci Technol. 2008. 43(10):1813–1823.
Article38. Song WY, Yang JA, Ku KH, Choi JH. Effect of red pepper seeds powder on antioxidative system and oxidative damage in rats fed high-fat.high cholesterol diet. J Korean Soc Food Sci Nutr. 2009. 38(9):1161–1166.
Article39. Joo HY, Lim KT. Protective effect of glycoprotein isolated from Cudrania tricuspidata on liver in CCl4-treated A/J mice. Korean J Food Sci Technol. 2009. 41(1):93–99.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of ethanol extracts from red pepper seeds on antioxidative defense system and oxidative stress in rats fed high-fat, high-cholesterol diet
- Effects of Liquid Culture of Coriolus Versicolor on Lipid Metabolism and Enzyme Activities in Rats Fed High Fat Diet
- Effects of Liquid Culture of Agaricus blazei Murill on Lipid Metabolism and Enzyme Activities in Rats Fed High Fat Diet
- Effect of Eisenia Bicyclis and Its Pill on Serum Lipid Status in Rats Fed High Fat Diet
- Effect of Seeds Extract of Paeonia Lactiflora on Antioxidative System and Lipid Peroxidation of Liver in Rats Fed High-Cholesterol Diet