Allergy Asthma Respir Dis.
2013 Mar;1(1):11-19. 10.4168/aard.2013.1.1.11.
Long-term asthma remission during adolescence
- Affiliations
-
- 1Department of Pediatrics, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea. kohyy@plaza.snu.ac.kr
- KMID: 2263054
- DOI: http://doi.org/10.4168/aard.2013.1.1.11
Abstract
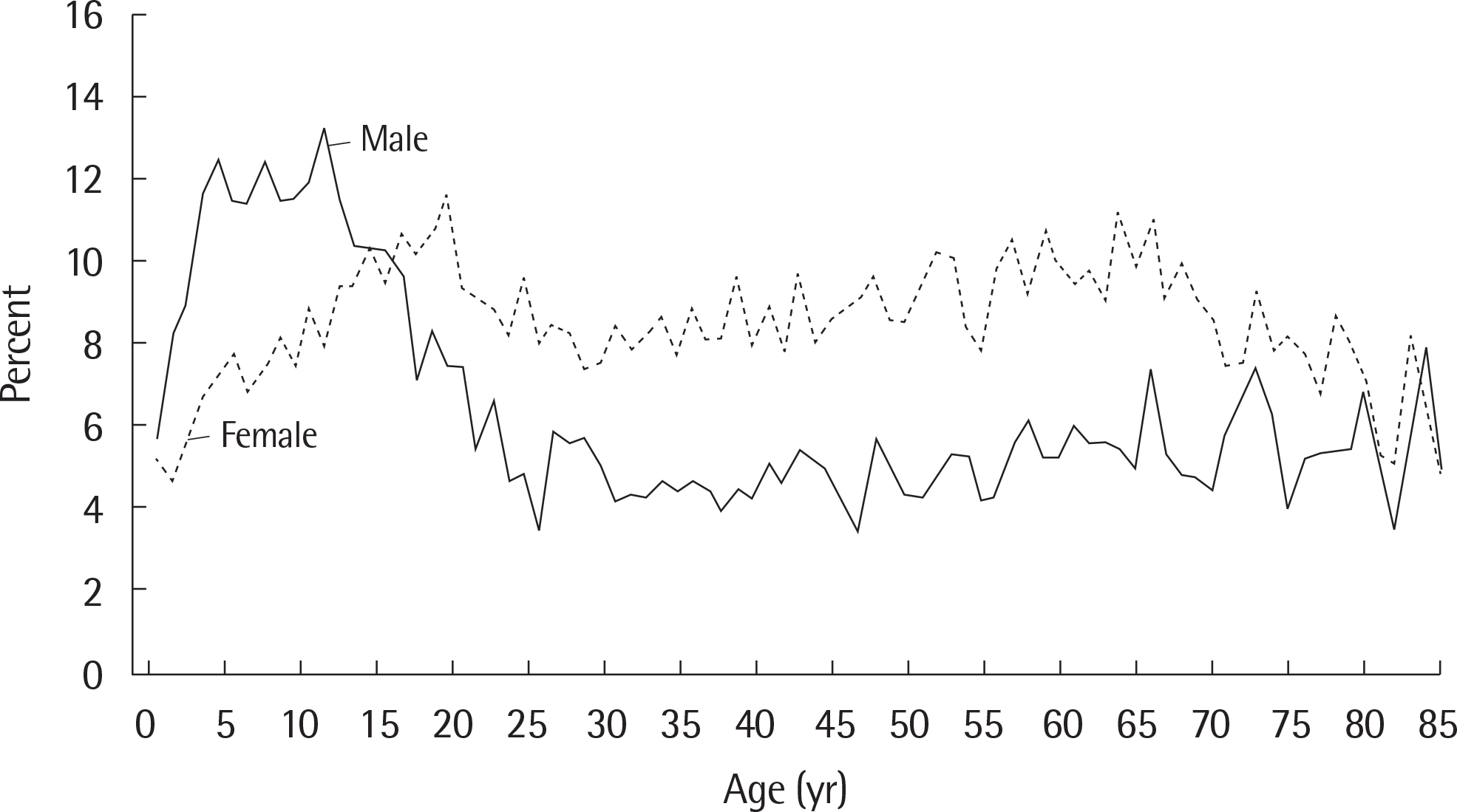
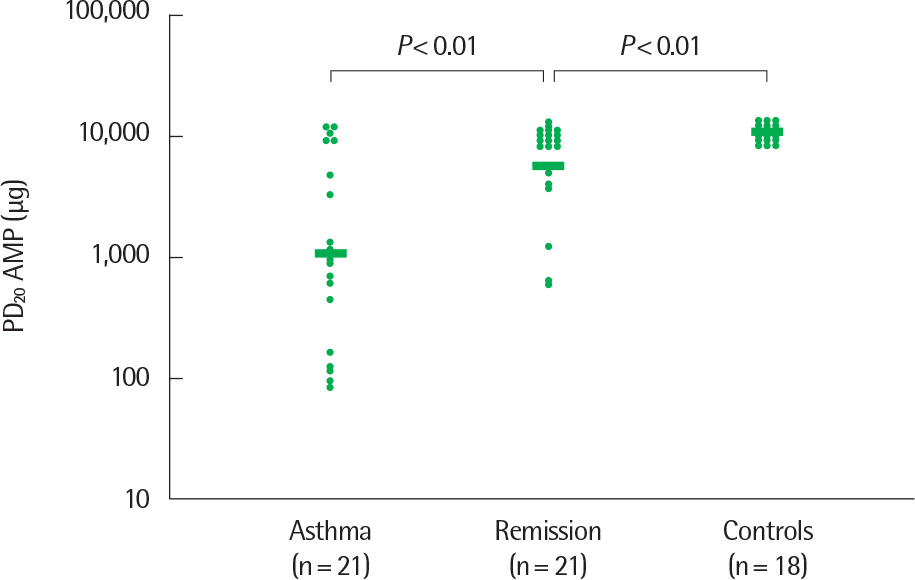
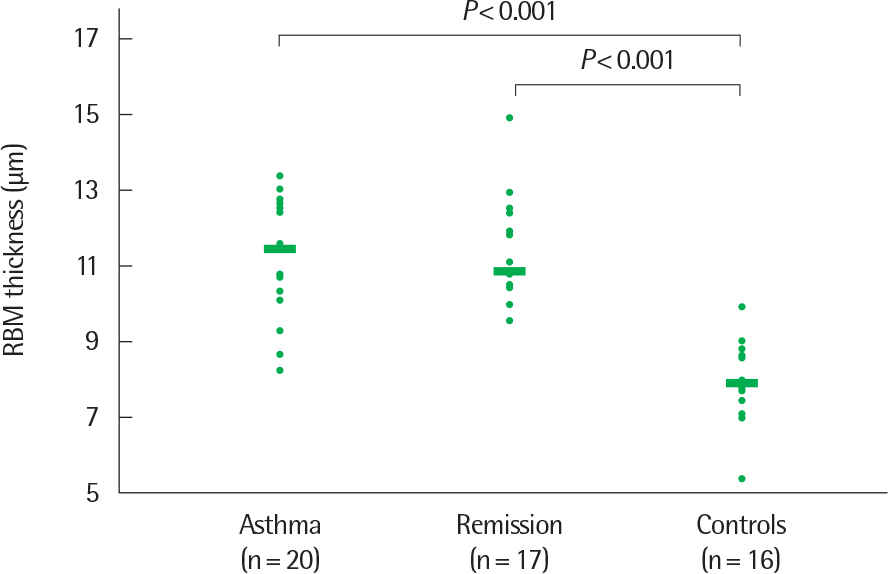
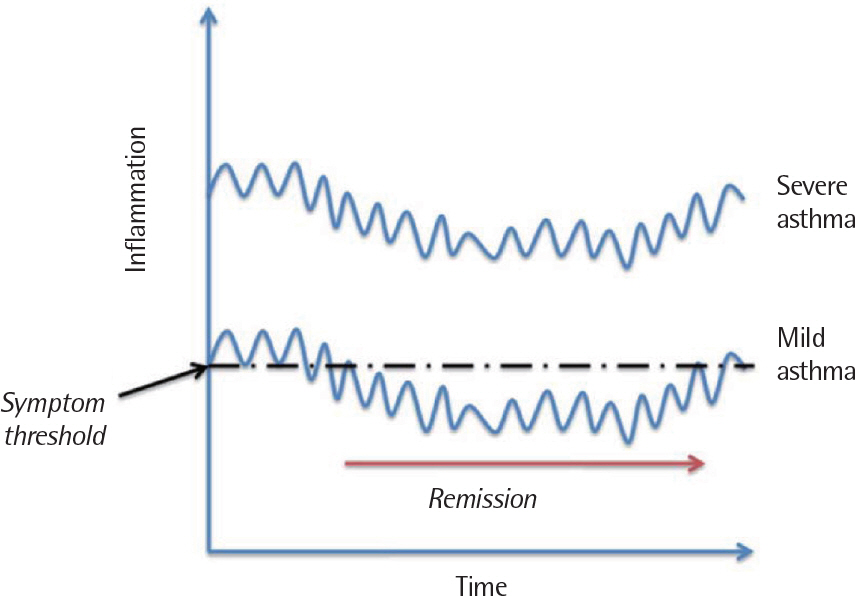
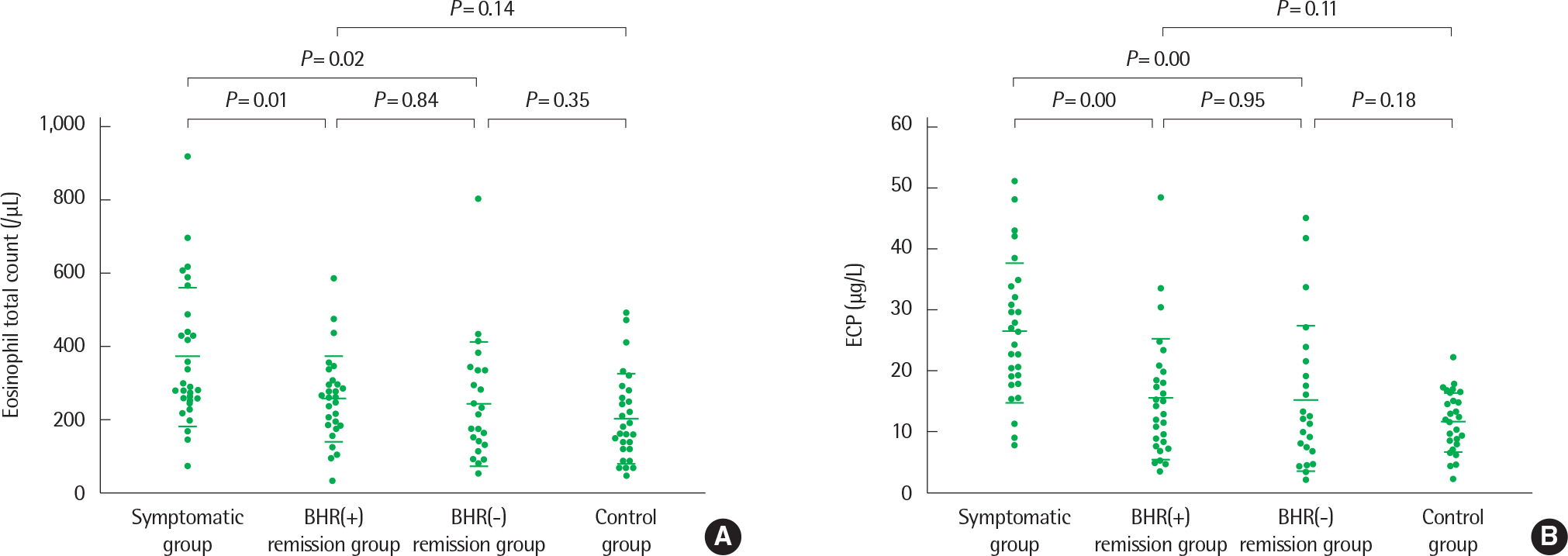
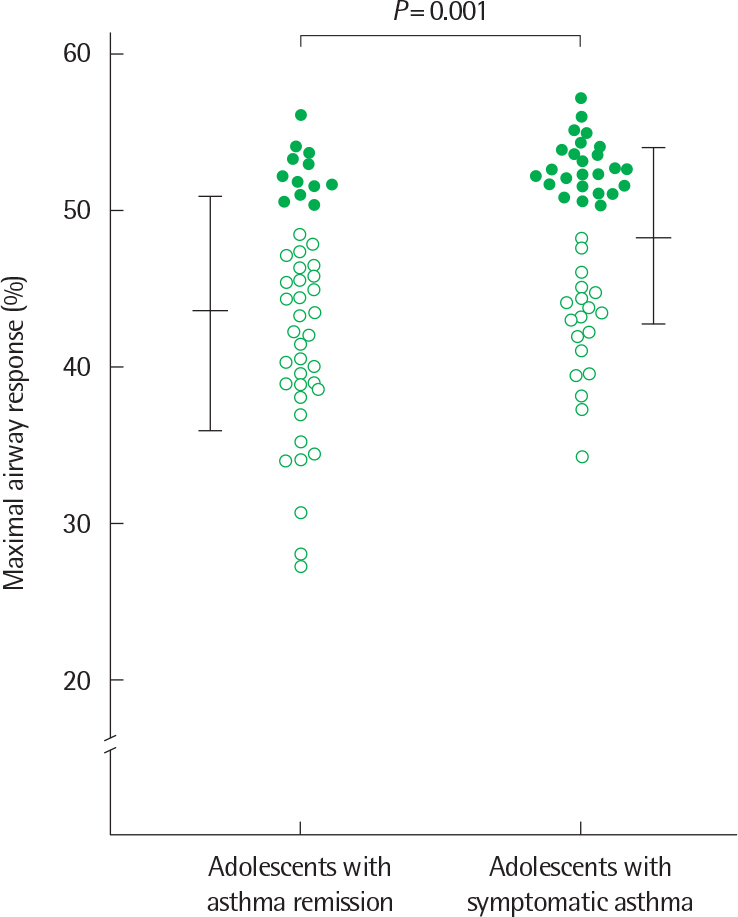
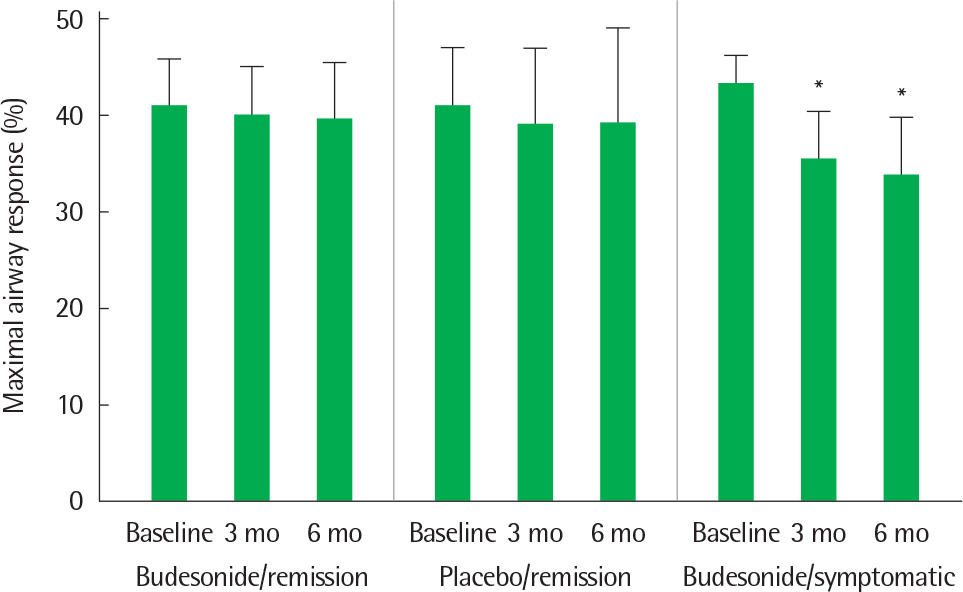
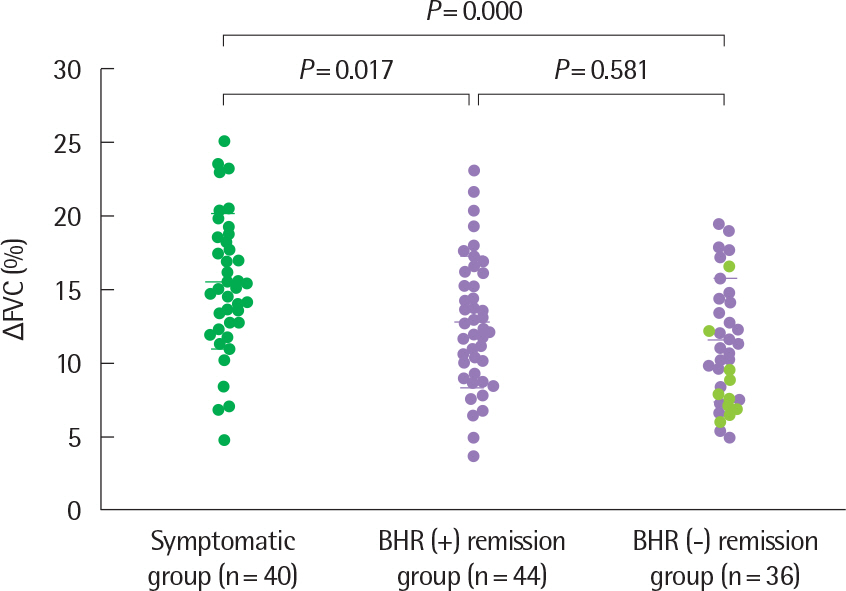
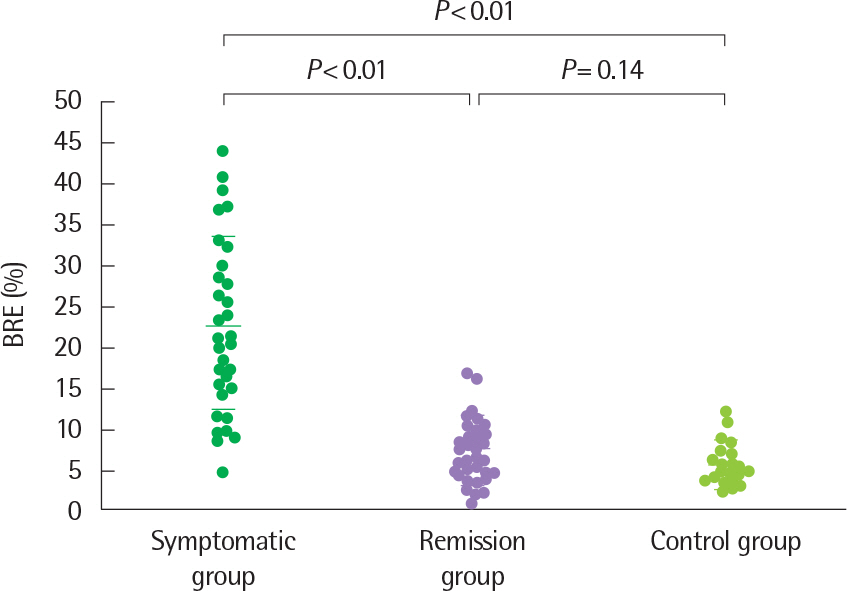
- Asthma is a heterogeneous disorder with a variable course, characterized by episodes of cough, wheezing and shortness of breath, reversible airflow limitation, and bronchial hyperresponsiveness (BHR). It begins early in life in many subjects, and it is well recognized that over 50% of asthmatic children go into long-term clinical remission, defined as the complete absence of asthmatic symptoms and no asthma medication for at least 24 months, during adolescence. Several studies have shown spirometric abnormalities and BHR during clinical remission. It is unknown whether these functional abnormalities, which are supposed to be indicative of asthma severity with respect to symptomatic asthma, reflect persistent airway inflammation or merely indicate residual airway damage or are related to another mechanism such as a familial predisposition. It is likely that the nature of BHR in asthma remission is not same as that in symptomatic asthma. We have shown that the former condition is associated with lower levels of blood eosinophils and eosinophilic cationic protein, a lower degree of bronchial responsiveness to exercise, and a more common formation of plateau on the dose-response curve to high-dose inhaled methacholine (i.e., limited maximal airway narrowing), compared to the latter condition. It is still controversial whether BHR in adolescents with asthma remission is reduced by inhaled corticosteroids. Better understanding of the mechanisms that lead to asthma remission, especially that seen during adolescence, is likely to lead to significant advances in our understanding of asthma pathogenesis, and should provide insights into how remission might be induced with therapy. We still have minimal understanding of the mechanism underlying BHR in adolescents with asthma remission. Elucidation of this mechanism would be an important step towards new perspectives that see remission as the next therapeutic frontier in asthma.
MeSH Terms
Figure
Reference
-
1. Upham JW, James AL. Remission of asthma: the next therapeutic fron-tier? Pharmacol Ther. 2011; 130:38–45.
Article2. Jenkins MA, Hopper JL, Bowes G, Carlin JB, Flander LB, Giles GG. Factors in childhood as predictors of asthma in adult life. BMJ. 1994; 309:90–3.
Article3. Kjellman B, Gustafsson PM. Asthma from childhood to adulthood: asthma severity, allergies, sensitization, living conditions, gender influence and social consequences. Respir Med. 2000; 94:454–65.
Article4. De Marco R, Locatelli F, Cerveri I, Bugiani M, Marinoni A, Giammanco G, et al. Incidence and remission of asthma: a retrospective study on the natural history of asthma in Italy. J Allergy Clin Immunol. 2002; 110:228–35.
Article5. Vonk JM, Postma DS, Boezen HM, Grol MH, Schouten JP, Koeter GH, et al. Childhood factors associated with asthma remission after 30 year follow up. Thorax. 2004; 59:925–9.
Article6. Martin AJ, McLennan LA, Landau LI, Phelan PD. The natural history of childhood asthma to adult life. Br Med J. 1980; 280:1397–400.
Article7. Guerra S, Wright AL, Morgan WJ, Sherrill DL, Holberg CJ, Martinez FD. Persistence of asthma symptoms during adolescence: role of obesity and age at the onset of puberty. Am J Respir Crit Care Med. 2004; 170:78–85.8. Taylor DR, Cowan JO, Greene JM, Willan AR, Sears MR. Asthma in remission: can relapse in early adulthood be predicted at 18 years of age? Chest. 2005; 127:845–50.9. Sekerel BE, Civelek E, Karabulut E, Yildirim S, Tuncer A, Adalioglu G. Are risk factors of childhood asthma predicting disease persistence in early adulthood different in the developing world? Allergy. 2006; 61:869–77.
Article10. To T, Gershon A, Wang C, Dell S, Cicutto L. Persistence and remission in childhood asthma: a population-based asthma birth cohort study. Arch Pediatr Adolesc Med. 2007; 161:1197–204.11. Covar RA, Strunk R, Zeiger RS, Wilson LA, Liu AH, Weiss S, et al. Pre-dictors of remitting, periodic, and persistent childhood asthma. J Allergy Clin Immunol. 2010; 125:359–66.e3.
Article12. Akinbami LJ, Moorman JE, Liu X. Asthma prevalence, health care use, and mortality: United States, 2005-2009. Natl Health Stat Report. 2011; 12:1–14.13. Sears MR, Greene JM, Willan AR, Wiecek EM, Taylor DR, Flannery EM, et al. A longitudinal, population-based, cohort study of childhood asthma followed to adulthood. N Engl J Med. 2003; 349:1414–22.
Article14. Nair P. Early interventions with inhaled corticosteroids in asthma: bene-fits and risks. Curr Opin Pulm Med. 2011; 17:12–5.
Article15. Boulet LP, Turcotte H, Brochu A. Persistence of airway obstruction and hyperresponsiveness in subjects with asthma remission. Chest. 1994; 105:1024–31.
Article16. van Den Toorn LM, Prins JB, Overbeek SE, Hoogsteden HC, de Jongste JC. Adolescents in clinical remission of atopic asthma have elevated exhaled nitric oxide levels and bronchial hyperresponsiveness. Am J Respir Crit Care Med. 2000; 162:953–7.
Article17. Obase Y, Shimoda T, Kawano T, Saeki S, Tomari S, Izaki K, et al. Bronchial hyperresponsiveness and airway inflammation in adolescents with asymptomatic childhood asthma. Allergy. 2003; 58:213–20.
Article18. Komatsu Y, Fujimoto K, Yasuo M, Urushihata K, Hanaoka M, Koizumi T, et al. Airway hyper-responsiveness in young adults with asthma that re-mitted either during or before adolescence. Respirology. 2009; 14:217–23.
Article19. Yoshikawa T, Kanazawa H. Phenotypic differences between asymptomatic airway hyperresponsiveness and remission of asthma. Respir Med. 2011; 105:24–30.
Article20. Gruber W, Eber E, Steinbrugger B, Modl M, Weinhandl E, Zach MS. Atopy, lung function and bronchial responsiveness in symptom-free paedi-atric asthma patients. Eur Respir J. 1997; 10:1041–5.
Article21. Panhuysen CI, Vonk JM, Koeter GH, Schouten JP, van Altena R, Bleecker ER, et al. Adult patients may outgrow their asthma: a 25-year follow-up study. Am J Respir Crit Care Med. 1997; 155:1267–72.
Article22. Broekema M, Timens W, Vonk JM, Volbeda F, Lodewijk ME, Hylkema MN, et al. Persisting remodeling and less airway wall eosinophil activation in complete remission of asthma. Am J Respir Crit Care Med. 2011; 183:310–6.
Article23. van den Toorn LM, Overbeek SE, de Jongste JC, Leman K, Hoogsteden HC, Prins JB. Airway inflammation is present during clinical remission of atopic asthma. Am J Respir Crit Care Med. 2001; 164:2107–13.
Article24. Hara J, Fujimura M, Myou S, Kita T, Abo M, Katayama N, et al. Sputum eosinophilia, airway hyperresponsiveness and airway narrowing in young adults with former asthma. Allergol Int. 2008; 57:211–7.
Article25. Volbeda F, ten Hacken NH, Lodewijk ME, Dijkstra A, Hylkema MN, Broekema M, et al. Can AMP induce sputum eosinophils, even in subjects with complete asthma remission? Respir Res. 2010; 11:106.
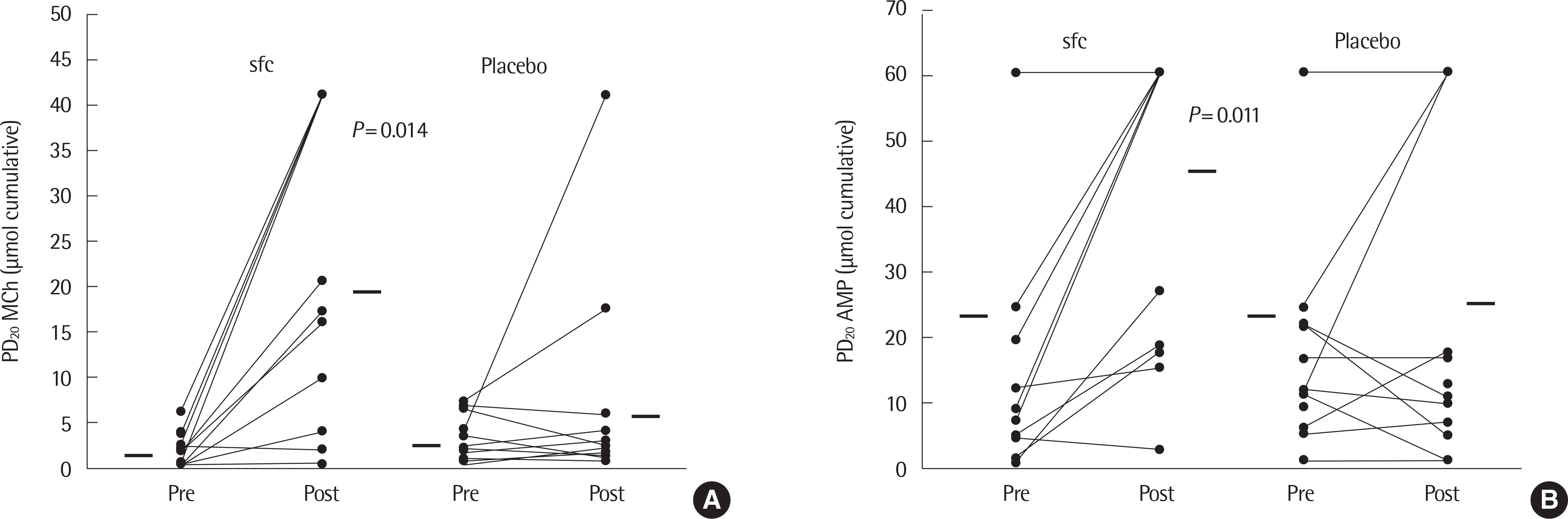
Article26. Koh YY, Sun YH, Lim HS, Kim CK, Hong SJ. Effect of inhaled budesonide on bronchial hyperresponsiveness in adolescents with clinical remission of asthma. Chest. 2001; 120:1140–6.
Article27. Koh YY, Kang EK, Kang H, Yoo Y, Park Y, Kim CK. Bronchial hyperresponsiveness in adolescents with long-term asthma remission: importance of a family history of bronchial hyperresponsiveness. Chest. 2003; 124:819–25.29. Tatar M, Petriskova J, Zucha J, Pecova R, Hutka Z, Raffajova J, et al. Induced sputum eosinophils, bronchial reactivity, and cough sensitivity in subjects with allergic rhinitis. J Physiol Pharmacol. 2005; 56(Suppl 4):227–36.30. Koh YY, Park Y, Kim CK. Maximal airway response in adolescents with long-term asthma remission and persisting airway hypersensitivity: its profile and the effect of inhaled corticosteroids. Chest. 2002; 122:1214–21.31. Yoo Y, Yu J, Kim DK, Koh YY. Percentage fall in FVC at the provocative concentration of methacholine causing a 20% fall in FEV1 in symptomatic asthma and clinical remission during adolescence. Chest. 2006; 129:272–7.
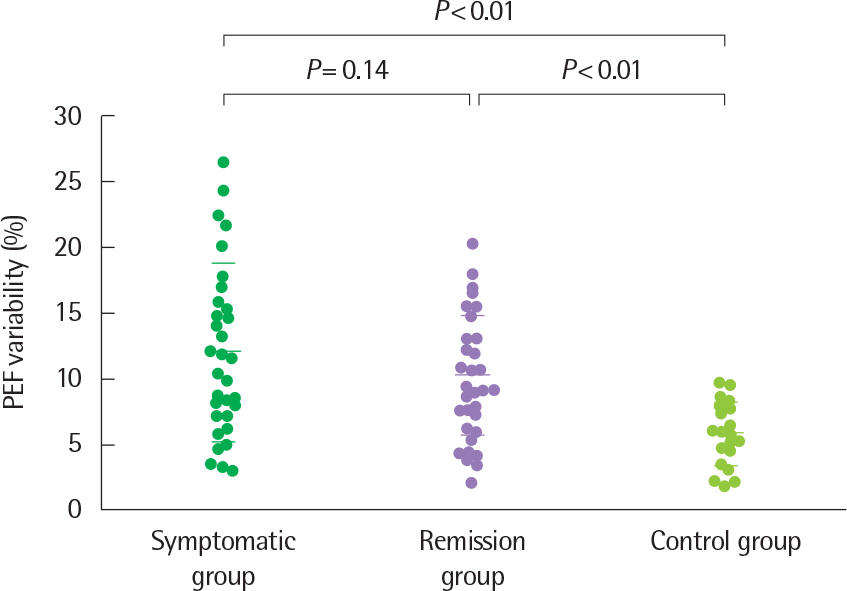
Article32. Koh YY, Kang H, Yoo Y, Yu J, Nah KM, Kim CK. Peak expiratory flow variability and exercise responsiveness in methacholine-hyperresponsive adolescents with asthma remission. J Asthma. 2005; 42:17–23.
Article33. Gutierrez V, Prieto L, Torres V, Morales C, Gonzalez E. Peak flow variability and sputum eosinophilia in allergic rhinitis. Ann Allergy Asthma Immunol. 1998; 81:143–50.34. Gibson PG, Mattoli S, Sears MR, Dolovich J, Hargreave FE. Increased peak flow variability in children with asymptomatic hyperresponsiveness. Eur Respir J. 1995; 8:1731–5.
Article35. Mochizuki H, Muramatsu R, Hagiwara S, Takami S, Mizuno T, Arakawa H. Relationship between bronchial hyperreactivity and asthma remission during adolescence. Ann Allergy Asthma Immunol. 2009; 103:201–5.
Article36. van den Toorn LM, Prins JB, de Jongste JC, Leman K, Mulder PG, Hoogsteden HC, et al. Benefit from anti-inflammatory treatment during clinical remission of atopic asthma. Respir Med. 2005; 99:779–87.
Article37. de Kluijver J, Evertse CE, Schrumpf JA, van der Veen H, Zwinderman AH, Hiemstra PS, et al. Asymptomatic worsening of airway inflammation during low-dose allergen exposure in asthma: protection by inhaled steroids. Am J Respir Crit Care Med. 2002; 166:294–300.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Peripheral Blood Eosinophil Counts and Serum ECP in Adolescents with Long-term Asthma Remission and Persistent Bronchial Hyperresponsiveness: Comparison with Adolescents with Symptomatic Asthma
- Analysis of the association between bronchial hyperresponsiveness and genetic polymorphism of beta2-adrenoceptor in adolescents with long-term asthma remission
- Predictive Factors for Valproate Treatment in Childhood Absence Epilepsy
- Pharmacologic Therapy of Asthma in Childhood
- Expert consensus on oral corticosteroid use and tapering in severe asthma management