Allergy Asthma Respir Dis.
2014 May;2(2):142-145. 10.4168/aard.2014.2.2.142.
Hypersensitivity reaction to aspirin accompanied by severe eosinophilia in a child with history of Kawasaki disease
- Affiliations
-
- 1Department of Pediatrics, Institute of Allergy, Severance Children's Hospital, Yonsei University College of Medicine, Seoul, Korea. kekim@yuhs.ac
- KMID: 2261751
- DOI: http://doi.org/10.4168/aard.2014.2.2.142
Abstract
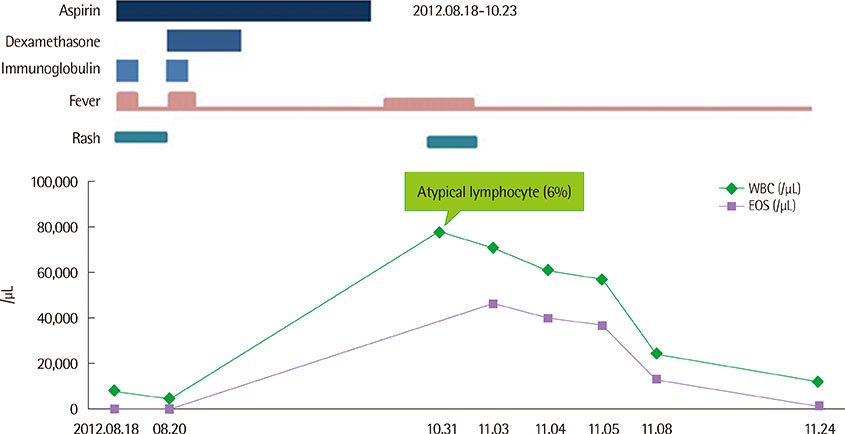
- Drug hypersensitivity is one of drug adverse reactions that develop in susceptible patients following exposure to certain drugs and cannot be predicted from the known pharmacology of a drug. Severe hypersensitivity is associated with high morbidity and mortality. Although the issue of hypersensitivity to nonsteroidal anti-inflammatory drugs (NSAIDs) has been largely investigated in adults, data related to NSAIDs hypersensitivity is insufficient in childhood. And in spite of the recommendation to avoid use of aspirin due to Reye syndrome in children, aspirin is one of major treatment along with intravenous immunoglobulin in Kawasaki disease. We report a case of a 10-month-old boy who underwent intravenous immunoglobulin and aspirin treatment for Kawasaki disease, and subsequently revealed severe leukocytosis and eosinophilia. To our knowledge, there have been no previous reports of aspirin-induced eosinophilia in Korea.
MeSH Terms
Figure
Reference
-
1. Khan DA, Solensky R. Drug allergy. J Allergy Clin Immunol. 2010; 125:2 Suppl 2. S126–S137.
Article2. Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA. 1998; 279:1200–1205.
Article3. Abonia JP, Castells M. Drug allergy in pediatric patients. Pediatr Ann. 2011; 40:200–204.
Article4. Sanchez-Borges M, Capriles-Behrens E, Caballero-Fonseca F. Hypersensitivity to non-steroidal anti-inflammatory drugs in childhood. Pediatr Allergy Immunol. 2004; 15:376–380.
Article5. Kidon MI, Liew WK, Chiang WC, Lim SH, Goh A, Tang JP, et al. Hypersensitivity to paracetamol in Asian children with early onset of nonsteroidal anti-inflammatory drug allergy. Int Arch Allergy Immunol. 2007; 144:51–56.
Article6. Kawakami T, Fujita A, Takeuchi S, Muto S, Soma Y. Drug-induced hypersensitivity syndrome: drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome induced by aspirin treatment of Kawasaki disease. J Am Acad Dermatol. 2009; 60:146–149.
Article7. Roufosse F, Weller PF. Practical approach to the patient with hypereosinophilia. J Allergy Clin Immunol. 2010; 126:39–44.
Article8. O'Byrne ML, Cohen MS. Marked eosinophilia in a patient with history of severe atypical Kawasaki disease. Congenit Heart Dis. 2013; 8:E130–E133.9. Valent P. Pathogenesis, classification, and therapy of eosinophilia and eosinophil disorders. Blood Rev. 2009; 23:157–165.
Article10. Brigden ML. A practical workup for eosinophilia. You can investigate the most likely causes right in your office. Postgrad Med. 1999; 105:193–194. 199–202. 207–210.11. Terai M, Yasukawa K, Honda T, Jibiki T, Hirano K, Sato J, et al. Peripheral blood eosinophilia and eosinophil accumulation in coronary microvessels in acute Kawasaki disease. Pediatr Infect Dis J. 2002; 21:777–781.
Article12. Kuo HC, Yang KD, Liang CD, Bong CN, Yu HR, Wang L, et al. The relationship of eosinophilia to intravenous immunoglobulin treatment failure in Kawasaki disease. Pediatr Allergy Immunol. 2007; 18:354–359.
Article13. Criado PR, Criado RF, Avancini Jde M, Santi CG. Drug reaction with eosinophilia and systemic symptoms (DRESS)/drug-induced hypersensitivity syndrome (DIHS): a review of current concepts. An Bras Dermatol. 2012; 87:435–449.
Article14. Song WJ, Chang YS. Drug hypersensitivity syndrome: a literature review of associated viral reactivation. Korean J Asthma Allergy Clin Immunol. 2011; 31:77–83.15. Peyriere H, Dereure O, Breton H, Demoly P, Cociglio M, Blayac JP, et al. Variability in the clinical pattern of cutaneous side-effects of drugs with systemic symptoms: does a DRESS syndrome really exist. Br J Dermatol. 2006; 155:422–428.
Article16. Aihara Y, Ito SI, Kobayashi Y, Yamakawa Y, Aihara M, Yokota S. Carbamazepine-induced hypersensitivity syndrome associated with transient hypogammaglobulinaemia and reactivation of human herpesvirus 6 infection demonstrated by real-time quantitative polymerase chain reaction. Br J Dermatol. 2003; 149:165–169.
Article17. Kim CW, Choi GS, Yun CH, Kim DI. Drug hypersensitivity to previously tolerated phenytoin by carbamazepine-induced DRESS syndrome. J Korean Med Sci. 2006; 21:768–772.
Article18. Kim YH, Choi CW, Lee GY, Kim WS. A case of valproic acid induced DRESS syndrome. Korean J Dermatol. 2012; 50:85–88.19. Shiohara T, Iijima M, Ikezawa Z, Hashimoto K. The diagnosis of a DRESS syndrome has been sufficiently established on the basis of typical clinical features and viral reactivations. Br J Dermatol. 2007; 156:1083–1084.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Reye-like Syndrome Combined with Kawasaki Disease
- Hypersensitivity to Aspirin and Nonsteroidal Anti-inflammatory Drugs
- A comparative study of therapeutic effect of combined treatment with aspirin and intravenous gammaglobulin versus aspirin alone in Kawasaki disease
- Drug Reaction with Eosinophilia and Systemic Symptom Syndrome Due to Everolimus: A Case Report
- A Case of Dieulafoy's Lesion Presenting Upper Gastrointestinal Bleeding in a Child in the Acute Phase of Kawasaki Disease