Korean J Hematol.
2006 Sep;41(3):199-203. 10.5045/kjh.2006.41.3.199.
A Case of Acute Lymphoblastic Leukemia in a Patient with Minimal Change Nephrotic Syndrome
- Affiliations
-
- 1Department of Internal Medicine, Pusan National University College of Medicine, Busan, Korea. hojinja@hanmail.net
- KMID: 2252302
- DOI: http://doi.org/10.5045/kjh.2006.41.3.199
Abstract
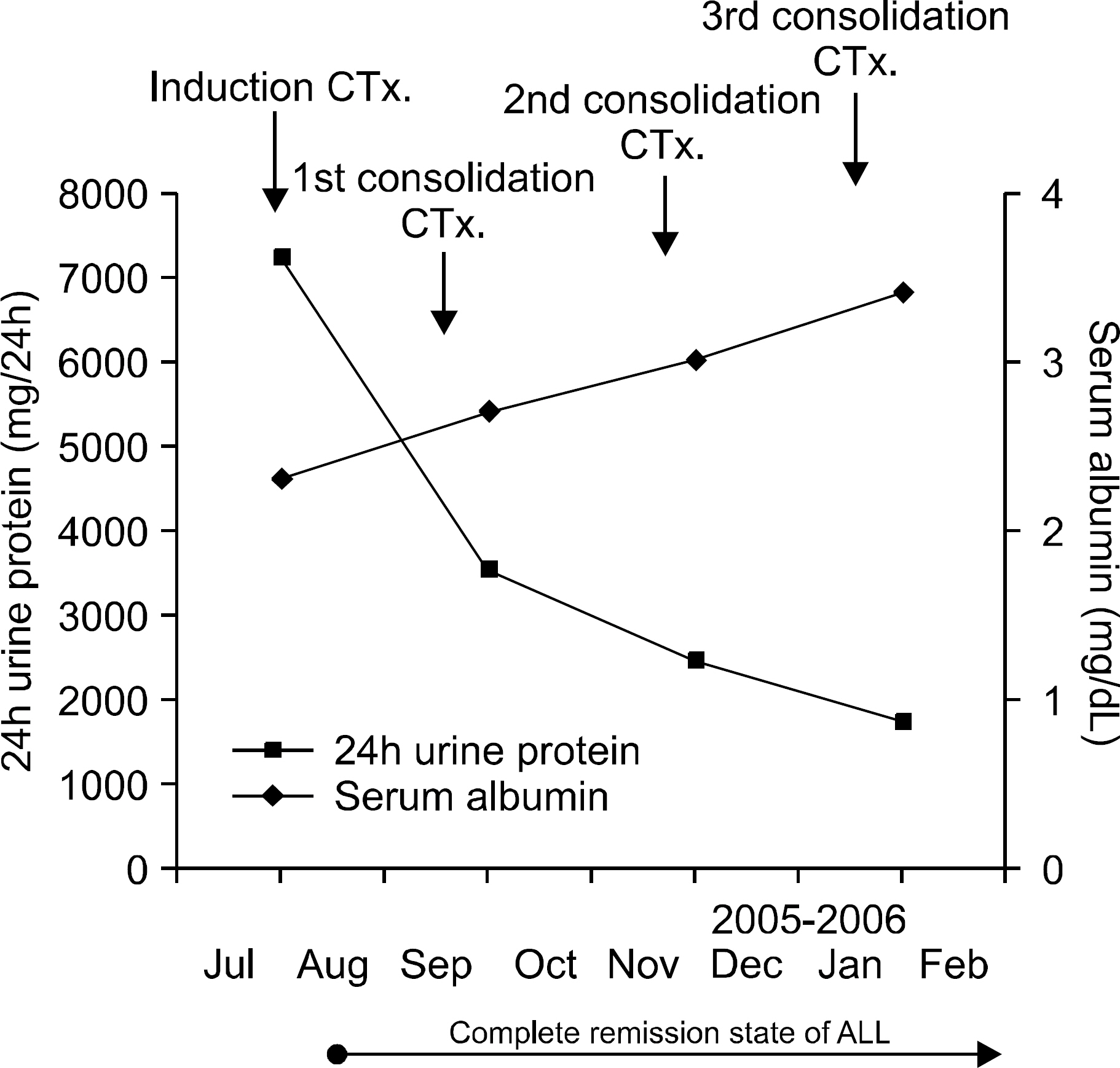
- We experienced a 22-year old patient with a documented history of minimal change nephrotic syndrome (MCNS), and a diagnosis of acute lymphoblastic leukemia (ALL) was then made for this patient. The patient received standard daily steroid therapy for the treatment of nephrotic syndrome. Cyclosporin A was administered because there was no clinical improvement with steroid therapy. Six years after the diagnosis of nephrotic syndrome, the patient was diagnosed with ALL. After chemotherapy for ALL, the patient was in complete remission and he showed clinical improvement of nephrotic syndrome. The hematological malignancies associated with nephrotic syndrome are mainly lymphoma and chronic lymphocytic leukemia. ALL has rarely been described in combination with nephrotic syndrome. Although the exact mechanism for development of ALL after nephrotic syndrome is unknown, at least two possibilities exist. First, the incidence of leukemia may be increased after immunosuppressive therapy, which may include cyclosporin A. Second, the underlying defect in T-lymphocyte function could account for both nephrotic syndrome and ALL. The possible mechanisms for such a relationship are discussed here along with a review of the relevant literature.
MeSH Terms
Figure
Reference
-
1). Galloway J. Remarks on Hodgkin's disease. Br Med J. 1922. 2:1201.
Article2). Levi I., Dinour D., Ben-Bassat I., Raanani P. Acute myeloid leukemia associated with nephrotic syndrome: case report and literature review. Leuk Lymphoma. 2002. 43:1133–6.
Article3). Mackie FE., Roy LP., Stevens M. Onset of leukaemia after levamisole treatment for nephrotic syndrome. Pediatr Nephrol. 1994. 8:527–8.
Article4). Rajpoot D., Tejani A., Rao S., Miller S. Focal segmental glomerulosclerosis in a child with acute lymphoblastic leukemia. Child Nephrol Urol. 1990. 10:231–3.5). Bhatia M., Kher K., Minniti CP. Acute lymphoblastic leukemia in a child with nephrotic syndrome. Pediatr Nephrol. 2004. 19:1290–3.
Article6). Ikeda Y., Sakemi T., Matsuzaki M., Sano M. Acute myelogenous leukemia following treatment with cyclosporin A in a nephrotic patient. Intern Med. 2002. 41:722–4.
Article7). Park JT., Kim JS., Kim HJ, et al. Clinical characteristics of nephrotic syndrome associated with malignancy. Korean J Nephrol. 2004. 23:738–45.8). Dabbs DJ., Striker LM., Mignon F., Striker G. Glomerular lesions in lymphomas and leukemias. Am J Med. 1986. 80:63–70.
Article9). Shalhoub RJ. Pathogenesis of lipoid nephrosis: a disorder of T-cell function. Lancet. 1974. 2:556–60.
Article10). Fiser RT., Arnold WC., Charlton RK., Steele RW., Chil dress SH., Shirkey B. T-lymphocyte subsets in nephrotic syndrome. Kidney Int. 1991. 40:913–6.
Article11). Zwanenburg TS., Suter W., Matter BE. Absence of genotoxic potential for cyclosporine in experimental systems. Transplant Proc. 1988. 20:931–3.12). Landewe RB., van den Borne BE., Breedveld FC., Dijkmans BA. Does cyclosporin A cause cancer? Nat Med. 1999. 5:714.
Article13). Paul C., Hornig F. Risk of malignancy associated with cyclosporin use in psoriasis. Dermatology. 1999. 198:320–1.14). Hojo M., Morimoto T., Maluccio M, et al. Cyclosporine induces cancer progression by a cell-autonomous mechanism. Nature. 1999. 397:530–4.
Article15). Bagga A., Mantan M. Nephrotic syndrome in children. Indian J Med Res. 2005. 122:13–28.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of the Minimal Change Nephropathy Associated with Small Cell Lung Cancer
- Precursor B-Cell Acute Lymphoblastic Leukemia in Two Patients with a History of Cytotoxic Therapy
- A Case of Minimal Change Nephrotic Syndrome Presented as Portal Vein Thrombosis and Acute Renal Failure
- A Case of Reversible Posterior Leukoencephalopathy in a Child with Acute Lymphoblastic Leukemia
- A case of acute lymphoblastic leukemia complicating neuroblastoma in remission