Korean J Hematol.
2011 Sep;46(3):186-191. 10.5045/kjh.2011.46.3.186.
New clinical score for disease activity at diagnosis in Langerhans cell histiocytosis
- Affiliations
-
- 1Department of Pediatrics, College of Medicine, Hanyang University Hospital, Seoul, Korea.
- 2Department of Surgery, University of Pittsburgh, Pittsburgh, PA, USA.
- 3Department of Laboratory Medicine & Genetics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 4Division of Pediatric Hematology/Oncology, Department of Pediatrics, University of Ulsan College of Medicine & Asan Medical Center, Seoul, Korea. jjseo@amc.seoul.kr
- KMID: 2252002
- DOI: http://doi.org/10.5045/kjh.2011.46.3.186
Abstract
- BACKGROUND
The clinical presentation and course of Langerhans cell histiocytosis (LCH) are variable, ranging from an isolated, spontaneously remitting bone lesion to multisystem disease with risk organ involvement. Treatment of LCH ranges from a wait-and-see attitude to intensive multidrug therapy and, in some cases, bone marrow transplantation. It is necessary to develop an objective score for assessing disease activity in patients with LCH. We propose a new clinical scoring system to evaluate disease activity at diagnosis that can predict the clinical outcomes of LCH and correlate it with clinical courses.
METHODS
Clinical data, obtained from children diagnosed with LCH at Asan Medical Center and Hanyang University Hospital between March 1998 and February 2009, were studied retrospectively. The scoring system was developed according to the basic biological data, radiological findings, and physical findings and applied to a database containing information on 133 patients.
RESULTS
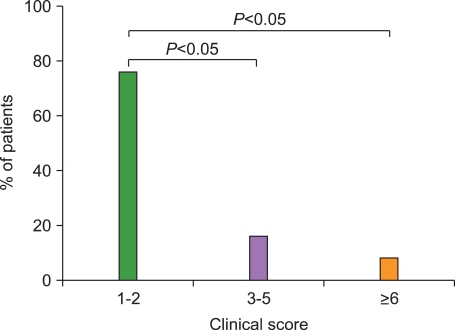
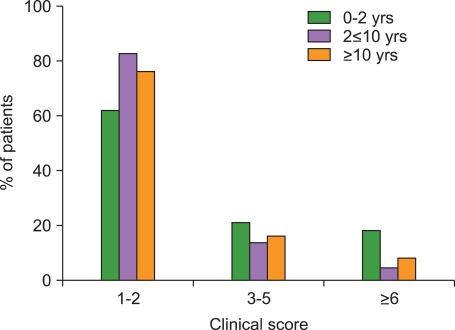
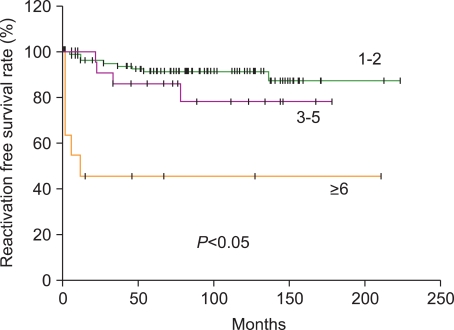
The median age of the 133 patients (74 male, 59 female) was 52 months (range, 0.6-178 months), and LCH was diagnosed based on CD1a positivity. At diagnosis, the score distributions were highly asymmetrical: the score was between 1 and 2 in 75.9% of cases, 3-6 in 15.8%, and greater than 6 in 8.3%. Initial scores above 6 were highly predictive of reactivation and late complications.
CONCLUSION
This new LCH disease activity score provides an objective tool for assessing disease severity, both at diagnosis and during follow-up.
MeSH Terms
Figure
Reference
-
1. Satter EK, High WA. Langerhans cell histiocytosis: a review of the current recommendations of the Histiocyte Society. Pediatr Dermatol. 2008; 25:291–295. PMID: 18577030.
Article2. Amir G, Weintraub M. Association of cell cycle-related gene products and NF-kappaB with clinical parameters in Langerhans cell histiocytosis. Pediatr Blood Cancer. 2008; 50:304–307. PMID: 17455317.
Article3. Weitzman S, Egeler RM. Langerhans cell histiocytosis: update for the pediatrician. Curr Opin Pediatr. 2008; 20:23–29. PMID: 18197035.
Article4. Gadner H, Grois N, Arico M, et al. A randomized trial of treatment for multisystem Langerhans' cell histiocytosis. J Pediatr. 2001; 138:728–734. PMID: 11343051.
Article5. Donadieu J, Piguet C, Bernard F, et al. A new clinical score for disease activity in Langerhans cell histiocytosis. Pediatr Blood Cancer. 2004; 43:770–776. PMID: 15390280.
Article6. Jubran RF, Marachelian A, Dorey F, Malogolowkin M. Predictors of outcome in children with Langerhans cell histiocytosis. Pediatr Blood Cancer. 2005; 45:37–42. PMID: 15768381.
Article7. Glotzbecker MP, Carpentieri DF, Dormans JP. Langerhans cell histiocytosis: clinical presentation, pathogenesis, and treatment from the LCH etiology research group at the Children's Hospital of Philadelphia. UPOJ. 2002; 15:67–73.8. Kilpatrick SE, Wenger DE, Gilchrist GS, Shives TC, Wollan PC, Unni KK. Langerhans' cell histiocytosis (histiocytosis X) of bone. A clinicopathologic analysis of 263 pediatric and adult cases. Cancer. 1995; 76:2471–2484. PMID: 8625073.
Article9. Alston RD, Tatevossian RG, McNally RJ, Kelsey A, Birch JM, Eden TO. Incidence and survival of childhood Langerhans' cell histiocytosis in Northwest England from 1954 to 1998. Pediatr Blood Cancer. 2007; 48:555–560. PMID: 16652350.
Article10. Satter EK, High WA. Langerhans cell histiocytosis: a review of the current recommendations of the Histiocyte Society. Pediatr Dermatol. 2008; 25:291–295. PMID: 18577030.
Article11. Weitzman S, Egeler RM. Langerhans cell histiocytosis: update for the pediatrician. Curr Opin Pediatr. 2008; 20:23–29. PMID: 18197035.
Article12. Bechan GI, Egeler RM, Arceci RJ. Biology of Langerhans cells and Langerhans cell histiocytosis. Int Rev Cytol. 2006; 254:1–43. PMID: 17147996.
Article13. Chikwava KR, Hunt JL, Mantha GS, Murphy JE, Jaffe R. Analysis of loss of heterozygosity in single-system and multisystem Langerhans' cell histiocytosis. Pediatr Dev Pathol. 2007; 10:18–24. PMID: 17378622.
Article14. Betts DR, Leibundgut KE, Feldges A, Plüss HJ, Niggli FK. Cytogenetic abnormalities in Langerhans cell histiocytosis. Br J Cancer. 1998; 77:552–555. PMID: 9484810.
Article15. Scappaticci S, Danesino C, Rossi E, et al. Cytogenetic abnormalities in PHA-stimulated lymphocytes from patients with Langerhans cell histocytosis. AIEOP-Istiocitosi Group. Br J Haematol. 2000; 111:258–262. PMID: 11091209.16. Schouten B, Egeler RM, Leenen PJ, Taminiau AH, van den Broek LJ, Hogendoorn PC. Expression of cell cycle-related gene products in Langerhans cell histiocytosis. J Pediatr Hematol Oncol. 2002; 24:727–732. PMID: 12468913.
Article17. Bank MI, Rengtved P, Carstensen H, Petersen BL. p53 expression in biopsies from children with Langerhans cell histiocytosis. J Pediatr Hematol Oncol. 2002; 24:733–736. PMID: 12468914.
Article18. Petersen BL, Rengtved P, Bank MI, Carstensen H. High expression of markers of apoptosis in Langerhans' cell histiocytosis. Histopathology. 2003; 42:186–193. PMID: 12558751.
Article19. Weintraub M, Bhatia KG, Chandra RS, Magrath IT, Ladisch S. p53 expression in Langerhans cell histiocytosis. J Pediatr Hematol Oncol. 1998; 20:12–17. PMID: 9482407.
Article20. Egeler RM, Favara BE, van Meurs M, Laman JD, Claassen E. Differential in situ cytokine profiles of Langerhans'-like cells and T cells in Langerhans' cell histiocytosis: abundant expression of cytokines relevant to disease and treatment. Blood. 1999; 94:4195–4201. PMID: 10590064.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Gastric Langerhans Cell Histiocytosis Showing Hypertrophic Folds
- A Case of Orbital Langerhans' cell histiocytosis
- Adult Scapular Langerhans Cell Histiocytosis Mistaken for Acute Osteomyelitis
- Spontaneous Pneumothorax due to Pulmonary Invasion in Multisystemic Langerhans Cell Histiocytosis: A case report
- A Case of Pulmonary Langerhans Cell Histiocytosis with Pneumothorax