J Gynecol Oncol.
2012 Jul;23(3):190-196. 10.3802/jgo.2012.23.3.190.
The effects of selenium on tumor growth in epithelial ovarian carcinoma
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. garden.lee@samsung.com
- KMID: 2245178
- DOI: http://doi.org/10.3802/jgo.2012.23.3.190
Abstract
OBJECTIVE
Epidemiological studies suggest that selenium protects against the development of several cancers. Selenium (sodium selenite) has been reported to interfere with cell growth and proliferation, and to induce cell death. In this study, we tested whether selenium could have growth-inhibiting effect in ovarian cancer cells and an orthotopic animal model.
METHODS
Cell growth in selenium-treated cells was determined in human ovarian cancer cells, A2780, HeyA8, and SKOV3ip1 using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide (MTT) assay. Animal experiment of selenium with paclitaxel was performed using SKOV3ip1 cells in nude mice to evaluate their inhibiting effect for tumor growth. In addition, another animal experiment of paclitaxel with or without selenium was performed to assess the effect of survival and food intake in mice.
RESULTS
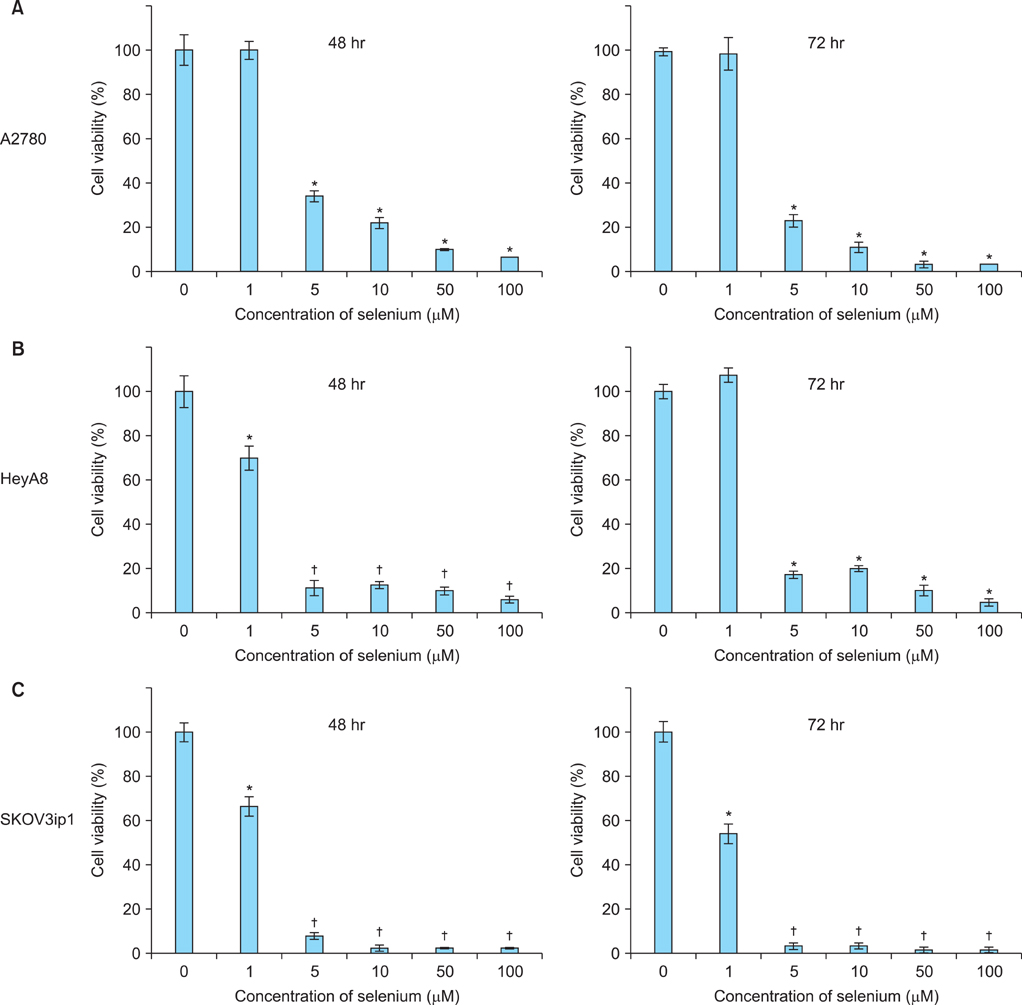
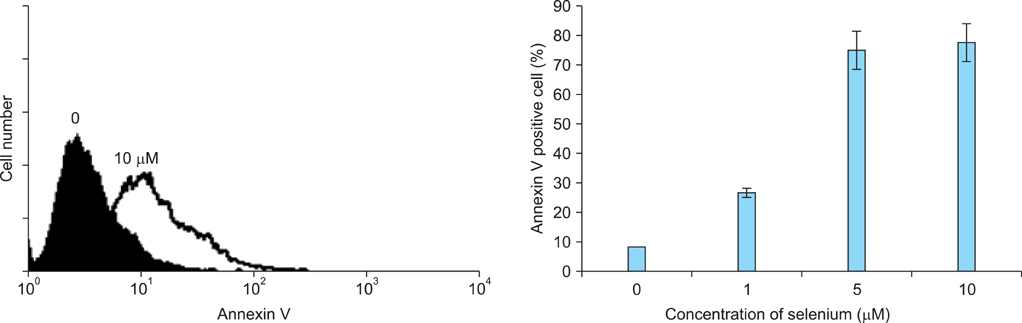
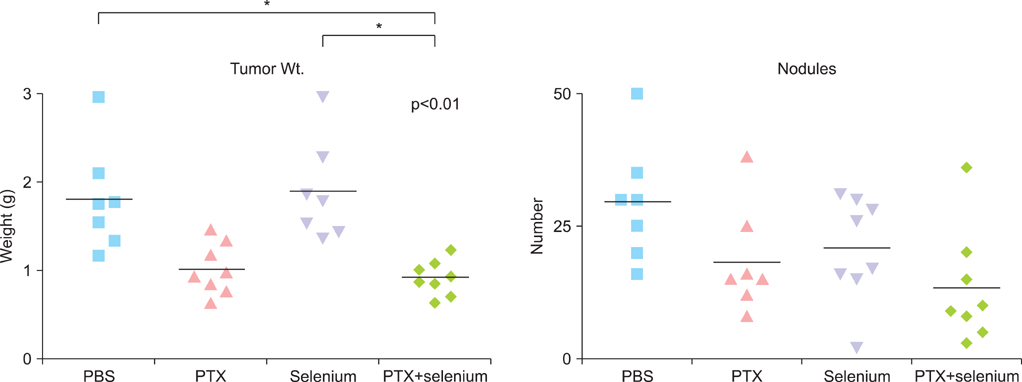
The in vitro growth of selenium-treated cells was significantly decreased dose-dependently in A2780, HeyA8, and SKOV3ip1 cells. Therapy experiment in mice was started 1 week after injection of the SKOV3ip1 cells. Treatment with selenium (1.5 mg/kg, 3 times/week) and paclitaxel injection showed no addictive effect of the inhibition of tumor growth. However, combination of selenium and paclitaxel showed the slightly increased food intake compared with paclitaxel alone.
CONCLUSION
Although selenium has growth-inhibiting effect in ovarian carcinoma cells in vitro, there is no additive effect on tumor growth in mice treated with combination of paclitaxel and selenium. However, food intake is slightly higher in selenium-treated mice during chemotherapy.
MeSH Terms
Figure
Reference
-
1. Landen CN Jr, Birrer MJ, Sood AK. Early events in the pathogenesis of epithelial ovarian cancer. J Clin Oncol. 2008. 26:995–1005.2. Ozols RF, Bookman MA, Connolly DC, Daly MB, Godwin AK, Schilder RJ, et al. Focus on epithelial ovarian cancer. Cancer Cell. 2004. 5:19–24.3. Clark LC, Combs GF Jr, Turnbull BW, Slate EH, Chalker DK, Chow J, et al. Nutritional Prevention of Cancer Study Group. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin: a randomized controlled trial. JAMA. 1996. 276:1957–1963.4. Mannisto S, Alfthan G, Virtanen M, Kataja V, Uusitupa M, Pietinen P. Toenail selenium and breast cancer-a case-control study in Finland. Eur J Clin Nutr. 2000. 54:98–103.5. Garland M, Morris JS, Stampfer MJ, Colditz GA, Spate VL, Baskett CK, et al. Prospective study of toenail selenium levels and cancer among women. J Natl Cancer Inst. 1995. 87:497–505.6. Wei WQ, Abnet CC, Qiao YL, Dawsey SM, Dong ZW, Sun XD, et al. Prospective study of serum selenium concentrations and esophageal and gastric cardia cancer, heart disease, stroke, and total death. Am J Clin Nutr. 2004. 79:80–85.7. Brooks JD, Metter EJ, Chan DW, Sokoll LJ, Landis P, Nelson WG, et al. Plasma selenium level before diagnosis and the risk of prostate cancer development. J Urol. 2001. 166:2034–2038.8. Helzlsouer KJ, Huang HY, Alberg AJ, Hoffman S, Burke A, Norkus EP, et al. Association between alpha-tocopherol, gamma-tocopherol, selenium, and subsequent prostate cancer. J Natl Cancer Inst. 2000. 92:2018–2023.9. Li H, Stampfer MJ, Giovannucci EL, Morris JS, Willett WC, Gaziano JM, et al. A prospective study of plasma selenium levels and prostate cancer risk. J Natl Cancer Inst. 2004. 96:696–703.10. Nomura AM, Lee J, Stemmermann GN, Combs GF Jr. Serum selenium and subsequent risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2000. 9:883–887.11. Yoshizawa K, Willett WC, Morris SJ, Stampfer MJ, Spiegelman D, Rimm EB, et al. Study of prediagnostic selenium level in toenails and the risk of advanced prostate cancer. J Natl Cancer Inst. 1998. 90:1219–1224.12. Papp LV, Lu J, Holmgren A, Khanna KK. From selenium to selenoproteins: synthesis, identity, and their role in human health. Antioxid Redox Signal. 2007. 9:775–806.13. Ganther HE. Selenium metabolism, selenoproteins and mechanisms of cancer prevention: complexities with thioredoxin reductase. Carcinogenesis. 1999. 20:1657–1666.14. Drake EN. Cancer chemoprevention: selenium as a prooxidant, not an antioxidant. Med Hypotheses. 2006. 67:318–322.15. Chen T, Wong YS, Zheng W, Bai Y, Huang L. Selenium nanoparticles fabricated in Undaria pinnatifida polysaccharide solutions induce mitochondria-mediated apoptosis in A375 human melanoma cells. Colloids Surf B Biointerfaces. 2008. 67:26–31.16. Dong H, Ying T, Li T, Cao T, Wang J, Yuan J, et al. Comparative proteomic analysis of apoptosis induced by sodium selenite in human acute promyelocytic leukemia NB4 cells. J Cell Biochem. 2006. 98:1495–1506.17. Jiang C, Hu H, Malewicz B, Wang Z, Lu J. Selenite-induced p53 Ser-15 phosphorylation and caspase-mediated apoptosis in LNCaP human prostate cancer cells. Mol Cancer Ther. 2004. 3:877–884.18. Kim YS, Jhon DY, Lee KY. Involvement of ROS and JNK1 in selenite-induced apoptosis in Chang liver cells. Exp Mol Med. 2004. 36:157–164.19. Lee JW, Shahzad MM, Lin YG, Armaiz-Pena G, Mangala LS, Han HD, et al. Surgical stress promotes tumor growth in ovarian carcinoma. Clin Cancer Res. 2009. 15:2695–2702.20. Lee JW, Han HD, Shahzad MM, Kim SW, Mangala LS, Nick AM, et al. EphA2 immunoconjugate as molecularly targeted chemotherapy for ovarian carcinoma. J Natl Cancer Inst. 2009. 101:1193–1205.21. Caffrey PB, Frenkel GD. Selenium compounds prevent the induction of drug resistance by cisplatin in human ovarian tumor xenografts in vivo. Cancer Chemother Pharmacol. 2000. 46:74–78.22. Combs GF Jr, Gray WP. Chemopreventive agents: selenium. Pharmacol Ther. 1998. 79:179–192.23. Combs GF Jr. Status of selenium in prostate cancer prevention. Br J Cancer. 2004. 91:195–199.24. Whanger PD. Selenium and its relationship to cancer: an update. Br J Nutr. 2004. 91:11–28.25. Han B, Wei W, Hua F, Cao T, Dong H, Yang T, et al. Requirement for ERK activity in sodium selenite-induced apoptosis of acute promyelocytic leukemia-derived NB4 cells. J Biochem Mol Biol. 2007. 40:196–204.26. Jonsson-Videsater K, Bjorkhem-Bergman L, Hossain A, Soderberg A, Eriksson LC, Paul C, et al. Selenite-induced apoptosis in doxorubicin-resistant cells and effects on the thioredoxin system. Biochem Pharmacol. 2004. 67:513–522.27. Kralova V, Brigulova K, Cervinka M, Rudolf E. Antiproliferative and cytotoxic effects of sodium selenite in human colon cancer cells. Toxicol In Vitro. 2009. 23:1497–1503.28. Zhong W, Oberley TD. Redox-mediated effects of selenium on apoptosis and cell cycle in the LNCaP human prostate cancer cell line. Cancer Res. 2001. 61:7071–7078.29. Zhao R, Xiang N, Domann FE, Zhong W. Expression of p53 enhances selenite-induced superoxide production and apoptosis in human prostate cancer cells. Cancer Res. 2006. 66:2296–2304.30. Zhao R, Domann FE, Zhong W. Apoptosis induced by selenomethionine and methioninase is superoxide mediated and p53 dependent in human prostate cancer cells. Mol Cancer Ther. 2006. 5:3275–3284.31. Cao S, Durrani FA, Rustum YM. Selective modulation of the therapeutic efficacy of anticancer drugs by selenium containing compounds against human tumor xenografts. Clin Cancer Res. 2004. 10:2561–2569.32. Corcoran NM, Najdovska M, Costello AJ. Inorganic selenium retards progression of experimental hormone refractory prostate cancer. J Urol. 2004. 171:907–910.33. Bhattacharyya RS, Husbeck B, Feldman D, Knox SJ. Selenite treatment inhibits LAPC-4 tumor growth and prostate-specific antigen secretion in a xenograft model of human prostate cancer. Int J Radiat Oncol Biol Phys. 2008. 72:935–940.34. Hu YJ, Chen Y, Zhang YQ, Zhou MZ, Song XM, Zhang BZ, et al. The protective role of selenium on the toxicity of cisplatin-contained chemotherapy regimen in cancer patients. Biol Trace Elem Res. 1997. 56:331–341.35. Sieja K, Talerczyk M. Selenium as an element in the treatment of ovarian cancer in women receiving chemotherapy. Gynecol Oncol. 2004. 93:320–327.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of c-Met in ovarian epithelial tumor
- Ovarian Borderline Epithelial Tumors
- Ovarian Large Cell Neuroendocrine Carcinoma Associated with Endocervical-like Mucinous Borderline Tumor: A Case Report and Literature Review
- Epithelial Ovarian Carcinoma: Analysis of Prognostic Factors
- Pharmacokinetics and cytotoxic effect of selenium compounds in rodent cancer xenograft model for therapy experiment