J Adv Prosthodont.
2014 Apr;6(2):115-120. 10.4047/jap.2014.6.2.115.
Effect of aging on tear strength and cytotoxicity of soft denture lining materials; in vitro
- Affiliations
-
- 1Graduate School of Clinical Dentistry, Institute for Clinical Dental Research, Korea University, Seoul, Republic of Korea. wddc@korea.ac.kr
- KMID: 2233339
- DOI: http://doi.org/10.4047/jap.2014.6.2.115
Abstract
- PURPOSE
The aim of this in vitro study was to evaluate the effect of aging on the tear strength and cytotoxicity of four soft denture lining materials.
MATERIALS AND METHODS
Four commonly used soft denture lining materials, (Coe-Comfort(TM) GC America Inc., Alsip, IL, USA; Coe-Soft(TM) GC America Inc., Alsip, IL, USA; Visco-gel Dentsply Caulk Milford, DE, USA; and Sofreliner Tough M Tokuyama Dental Corporation Tokyo, Japan) were selected. Sixty trouser-leg designed specimens per lining material were fabricated using a stainless steel mold for tear strength testing. The specimens were divided into non-thermocycling and 1000-, and 3000- thermocycling groups. For the cytotoxicity test, twenty-four disk shaped specimens per material were fabricated using a stainless steel mold. The specimens were soaked in normal saline solution for 24 h, 48 h and 72 h. Cytotoxicity was measured by XTT assay in L929 mouse fibroblasts. Data were analyzed by two way analysis of variance and Dunnett's test (P<.05).
RESULTS
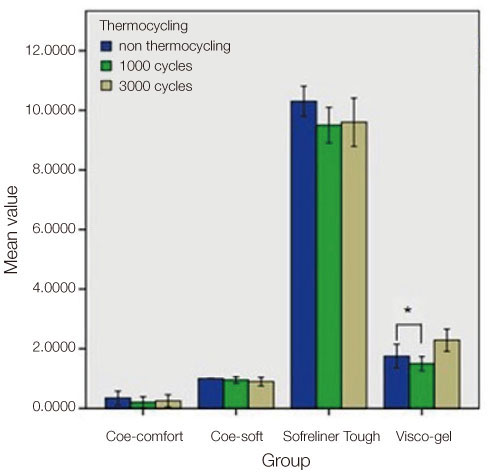
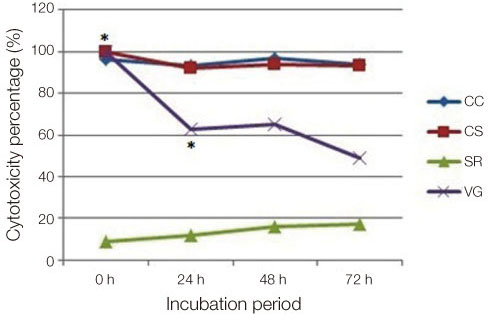
Before thermocycling, Sofreliner Tough M (10.36 +/- 1.00 N) had the highest tear strength value while Coe-Comfort(TM) (0.46 +/- 0.10 N) had the lowest. After 3000 cycles, Sofreliner Tough M (9.65 +/- 1.66 N) presented the highest value and Coe-Comfort(TM) (0.42 +/- 0.08 N) the lowest. Sofreliner Tough M, in all incubation periods was the least toxic with significant differences compared to all other materials (P<.05). Coe-Comfort(TM), Coe-Soft(TM), and Sofreliner Tough M did not show any significant differences within their material group for all incubation periods.
CONCLUSION
This in vitro study revealed that aging can affect both the tear strength and cytotoxicity of soft denture materials depending on the composition.
MeSH Terms
Figure
Reference
-
1. Sertgöz A, Kulak Y, Gedik H, Taskonak B. The effect of thermocycling on peel strength of six soft lining materials. J Oral Rehabil. 2002; 29:583–587.2. Pesun IJ, Hodges J, Lai JH. Effect of finishing and polishing procedures on the gap width between a denture base resin and two long-term, resilient denture liners. J Prosthet Dent. 2002; 87:311–318.3. Murphy WM, Huggett R, Handley RW, Brooks SC. Rigid cold curing resins for direct use in the oral cavity. Br Dent J. 1986; 160:391–394.4. Qudah S, Harrison A, Huggett R. Soft lining materials in prosthetic dentistry: a review. Int J Prosthodont. 1990; 3:477–483.5. Anusavice KJ. Phillips' science of dental materials. St. Louis; Mo.: WB Saunders;2003.6. Causton BE. Denture base polymers and liners. In : O'Brian WJ, editor. Dental materials and their selection. Chicago: Quintessence;1997. p. 90–92.7. Ozdemir KG, Yilmaz H, Yilmaz S. In vitro evaluation of cytotoxicity of soft lining materials on L929 cells by MTT assay. J Biomed Mater Res B Appl Biomater. 2009; 90:82–86.8. Shanmuganathan N, Padamanabhan TV, Subramaniam R, Madhankumar S. The compliance of temporary soft lining materials-an in vivo & vitro study. Int J Sci Res Publ. 2012; 2:1–7.9. Polyzois GL, Hensten-Pettersen A, Kullmann A. An assessment of the physical properties and biocompatibility of three silicone elastomers. J Prosthet Dent. 1994; 71:500–504.10. Park SK, Lee YK, Lim BS, Kim CW. Changes in properties of short-term-use soft liners after thermocycling. J Oral Rehabil. 2004; 31:717–724.11. Chaves CA, Machado AL, Vergani CE, de Souza RF, Giampaolo ET. Cytotoxicity of denture base and hard chairside reline materials: a systematic review. J Prosthet Dent. 2012; 107:114–127.12. Munksgaard EC. Leaching of plasticizers from temporary denture soft lining materials. Eur J Oral Sci. 2004; 112:101–104.13. International Standards Organization (ISO). 10993-5:1992. Biological evaluation of medical devices - Part 5: Tests for in vitro cytotoxicity. Geneva; Switzerland: International Standards Organization;1992.14. Hensten-Pettersen A. Comparison of the methods available for assessing cytotoxicity. Int Endod J. 1988; 21:89–99.15. Heravi F, Ramezani M, Poosti M, Hosseini M, Shajiei A, Ahrari F. In Vitro Cytotoxicity Assessment of an Orthodontic Composite Containing Titanium-dioxide Nano-particles. J Dent Res Dent Clin Dent Prospects. 2013; 7:192–198.16. Jepson NJ, McCabe JF, Storer R. Evaluation of the viscoelastic properties of denture soft lining materials. J Dent. 1993; 21:163–170.17. Lytle RB. Complete denture construction based on a study of the deformation of the underlying soft tissues. J Prosthet Dent. 1959; 9:539–551.18. Mjör IA. Biologic assessment of restorative dental materials: interrelationship of biologic and technologic properties. Oper Dent. 1978; 3:9–13.19. Huang FM, Tai KW, Hu CC, Chang YC. Cytotoxic effects of denture base materials on a permanent human oral epithelial cell line and on primary human oral fibroblasts in vitro. Int J Prosthodont. 2001; 14:439–443.20. Lefebvre CA, Schuster GS. Biocompatibility of visible lightcured resin systems in prosthodontics. J Prosthet Dent. 1994; 71:178–185.21. Hanks CT, Strawn SE, Wataha JC, Craig RG. Cytotoxic effects of resin components on cultured mammalian fibroblasts. J Dent Res. 1991; 70:1450–1455.22. International Standards Organization (ISO). ISO 7405:1997. Pre clinical evaluation of biocompatibility of medical devices used in dentistry: Test methods for dental materials. Geneva; Switzerland: International Standards Organization;1997.23. Thonemann B, Schmalz G, Hiller KA, Schweikl H. Responses of L929 mouse fibroblasts, primary and immortalized bovine dental papilla-derived cell lines to dental resin components. Dent Mater. 2002; 18:318–323.24. Bouillaguet S, Shaw L, Gonzalez L, Wataha JC, Krejci I. Long-term cytotoxicity of resin-based dental restorative materials. J Oral Rehabil. 2002; 29:7–13.25. Roehm NW, Rodgers GH, Hatfield SM, Glasebrook AL. An improved colorimetric assay for cell proliferation and viability utilizing the tetrazolium salt XTT. J Immunol Methods. 1991; 142:257–265.26. Stevens MG, Olsen SC. Comparative analysis of using MTT and XTT in colorimetric assays for quantitating bovine neutrophil bactericidal activity. J Immunol Methods. 1993; 157:225–231.27. Dootz ER, Koran A, Craig RG. Physical property comparison of 11 soft denture lining materials as a function of accelerated aging. J Prosthet Dent. 1993; 69:114–119.28. Hekimoğlu C, Anil N. The effect of accelerated ageing on the mechanical properties of soft denture lining materials. J Oral Rehabil. 1999; 26:745–748.29. León BL, Del Bel Cury AA, Rodrigues Garcia RC. Water sorption, solubility, and tensile bond strength of resilient denture lining materials polymerized by different methods after thermal cycling. J Prosthet Dent. 2005; 93:282–287.30. Pinto JR, Mesquita MF, Nóbilo MA, Henriques GE. Evaluation of varying amounts of thermal cycling on bond strength and permanent deformation of two resilient denture liners. J Prosthet Dent. 2004; 92:288–293.31. Qudah S, Huggett R, Harrison A. The effect of thermocycling on the hardness of soft lining materials. Quintessence Int. 1991; 22:575–580.32. Oguz S, Mutluay MM, Dogan OM, Bek B. Effect of thermocycling on tensile strength and tear resistance of four soft denture liners. Dent Mater J. 2007; 26:296–302.33. Murata H, Iwanaga H, Shigeto N, Hamada T. Initial flow of tissue conditioners-influence of composition and structure on gelation. J Oral Rehabil. 1993; 20:177–187.34. Ciapetti G, Granchi D, Stea S, Savarino L, Verri E, Gori A, Savioli F, Montanaro L. Cytotoxicity testing of materials with limited in vivo exposure is affected by the duration of cell-material contact. J Biomed Mater Res. 1998; 42:485–490.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- INHIBITORY EFFECT OF ANTIFUNGAL AGENTS INCORPORATED IN DENTURE LINING MATERIALS AGAINST CANDIDA ALBICANS
- In vitro study on the adherence and penetration of candida albicans into denture soft lining materials
- THE TENSLE BOND STRENGTH AND ELASTIC MODULUS OF THE SOFT DENTURE LINING MATERIALS
- The effect of denture cleansers on soft lining materials
- A study on the adhesion of a soft liner containing 4-META to the base metal alloy and its viscoelastic property