Korean Circ J.
2007 Jan;37(1):27-32. 10.4070/kcj.2007.37.1.27.
Increased Inflammatory Markers and Endothelial Dysfunction are Associated with Variant Angina
- Affiliations
-
- 1The Heart Center of Chonnam National University Hospital, Gwangju, Korea. myungho@chollian.net
- 2College of Nursing of Chonnam National University, Gwangju, Korea.
- 3Chonnam National University Research Institutute of Medical Sciences, Gwangju, Korea.
- KMID: 2227090
- DOI: http://doi.org/10.4070/kcj.2007.37.1.27
Abstract
-
BACKGROUND AND OBJECTIVES: Endothelial dysfunction and increased vascular inflammation may be associated with variant angina (VA). However, their exact roles remain to be clarified. The aim of the presents study is to investigate whether the level of inflammation markers and the flow-mediated dilation (FMD) are related to VA.
SUBJECTS AND METHODS
The study included 46 patients (VA group: 53.9+/-12.0 years, 20 males) with positive spasm provocation tests and they were without significant coronary stenosis, and 14 patients (control group: 46.6+/-13.5 years, 7 males) with negative spasm provocation tests and they were without significant coronary stenosis. The clinical characteristics and inflammatory markers, including the high sensitive C-reactive protein (hsCRP) level, the monocyte count and the von Willebrand factor (vWF) level, and the FMD were compared between the two groups. The FMD and inflammatory markers were measured in the morning before performing the ergonovine provocation coronary angiogram.
RESULTS
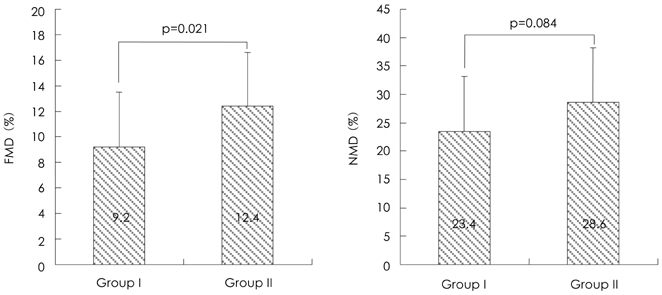
The level of vWF was significantly higher in the VA group than in the control group (166.5+/-41.9% vs. 118.0+/-65.3%, respectively, p=0.029). The FMD was significantly decreased in the VA group compared with the control group (9.2+/-4.3% vs. 12.4+/-4.2%, respectively, p=0.021). Nitrate-mediated dilation did not differ between the two groups. The levels of the monocyte count, hs-CRP and homocysteine were higher in the VA group than in the control group (554.7+/-261.0/mm3 vs. 440.7+/-136.0/mm3, respectively, p=0.039; 0.3+/-0.4 mg/dL vs. 0.1+/-0.1 mg/dL, respectively, p=0.029; 7.54+/-4.0micronmol/L vs. 5.92+/-1.6micronmol/L, respectively, p=0.033).
CONCLUSION
The results of this study suggested that increased inflammatory markers and endothelial dysfunction may be associated with variant angina.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Usefulness of Plasma Von Willebrand Factor and Brachial Artery Endothelial Dysfunction to Predict Variant Angina
Sook Hee Cho, In Hyae Park, Myung Ho Jeong, Jin Soo Choi, Hyun Ju Yun, Kye Hun Kim, Young Joon Hong, Hyung Wook Park, Ju Han Kim, Youngkeun Ahn, Jeong Gwan Cho, Jong Chun Park, Jung Chae Kang
Chonnam Med J. 2008;44(2):65-71. doi: 10.4068/cmj.2008.44.2.65.
Reference
-
1. Zipes DP, Libby P, Bonow RO, Braunwald E. Braunwald's Heart Disease. 2005. 7th ed. Philadelphia: WB Saunders;1264–1267.2. Kang JA, Lee YS, Jeong SH, et al. Clinical characteristics of patients with variant angina. Korean J Med. 2002. 63:195–202.3. Jeong MH, Park JC, Rhew JY, et al. Successful management of intractable coronary spasm with a coronary stent. Jpn Circ J. 2000. 64:897–900.4. Yasue H, Kugiyama K. Coronary spasm: clinical features and pathogenesis. Intern Med. 1997. 36:760–765.5. Yamagishi M, Terashima M, Awano K, et al. Morphology of vulnerable coronary plaque: insights from follow-up of patients examined by intravascular ultrasound before an acute coronary syndrome. J Am Coll Cardiol. 2000. 35:106–111.6. Saito S, Yamagishi M, Takayama T, et al. Plaque morphology at coronary sites with focal spasm in variant angina: study using intravascular ultrasound. Circ J. 2003. 67:1041–1045.7. Lee GR, Bithell TC, Foerster J. Wintrobe's Clinical Hematology. 1993. 9th ed. Philadelphia: Lea & Febriger;575–577.8. Roldan V, Marin F, Garcia-Herola A, Lip GY. Correlation of plasma von Willebrand factor levels, an index of endothelial damage/ dysfunction, with two point-based stroke risk stratification scores in atrial fibrillation. Thromb Res. 2005. 116:321–325.9. Fowkes FG, Lowe GD, Housely E, et al. Cross linked fibrin degradation products, progression of peripheral arterial disease, and risk of coronary heart disease. Lancet. 1993. 342:84–86.10. Celermajer DS, Sorensen KE, Gooch VM, et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992. 340:1111–1115.11. Hung MJ, Cherng WJ, Cheng CW, Li LF. Comparison of serum levels of inflammatory markers in patients with coronary vasospasm without significant fixed coronary artery disease versus patients with stable angina pectoris and acute coronary syndromes with significant fixed coronary artery disease. Am J Cardiol. 2006. 97:1429–1434.12. Maseri A, Severi S, Nes MD, et al. Variant angina: one aspect of a continuous spectrum of vasospastic myocardial ischemia: pathologenetic mechanisms, estimated incidence and clinical and coronary arteriographic findings in 138 patients. Am J Cardiol. 1978. 42:1019–1035.13. Chahine RA, Raizner AE, Ishimori T, Luchin RJ, McIntosh HD. The incidence and clinical implications of coronary arterty spasm. Circulation. 1975. 52:972–978.14. Yasue H, Omote S, Takizawa A, Nagao M, Miwa K, Tanaka S. Circadian variation of exercise capacity in patients with Prinzmetal's variant angina: role of exercise-induced coronary arterial spasm. Circulation. 1979. 59:938–948.15. Kugiyama K, Yasue H, Okumura K, et al. Nitric oxide activity is deficient in spasm arteries of patients with coronary spastic angina. Circulation. 1996. 94:266–271.16. Teragawa H, Kato M, Kurokawa J, Yamagata T, Matsuura H, Chayama K. Endothelial dysfunction is an independent factor responsible for vasospastic angina. Clin Sci. 2001. 101:707–713.17. Yamamoto H. Preserved endothelial function in the spastic segment of the human epicardial coronary artery in patients with variant angina: role of substance P in evaluating endothelial function. Eur Heart J. 1993. 14:Suppl I. 118–122.18. Botker HE, Sonne HS, Sorensen KE. Frequency of systemic microvascular dysfunction in syndrome X and in variant angina. Am J Cardiol. 1996. 78:182–186.19. Motoyama T, Kawano H, Kugiyama K, et al. Flow-mediated, endothelium-dependent dilation of the brachial arteries is impaired in patients with coronary spastic angina. Am Heart J. 1997. 133:263–267.20. Motoyama T, Kawano H, Kugiyama K, et al. Vitamin E admini-stration improves impairment of endothelium-dependent vasodilation in patients with coronary spastic angina. J Am Coll Cardiol. 1998. 32:1672–1679.21. Hirashima O, Kawano H, Motoyama T, et al. Improvement of endothelial function and insulin sensitivity with vitamin C in patients with coronary spastic angina: possible role of reactive oxygen species. J Am Coll Cardiol. 2000. 35:1860–1866.22. Choi MJ, Lee YS, Choi JS, et al. The clinical significance of smoking in Korean patients with variant angina. J Korean Soc Hypertension. 2004. 10:8–14.23. Han KI, Han KH, Park SW, et al. The vasomotor tone in vasospastic angina. Korean Circ J. 1991. 21:889–896.24. Kim NH, Jeong JW, Park EM, et al. Alterations of autonomic nervous activity associated with spontaneous coronary spasm in patients with variant angina. Korean Circ J. 2004. 34:362–367.25. Libby P, Ridker PM. Inflammation and atherosclerosis: role of C-reactive protein in risk assessment. Am J Med. 2004. 116:9S–16S.26. Devaraj S, Kumaresan PR, Jialal I. Effect of C-reactive protein on chemokine expression in human aortic endothelial cells. J Mol Cell Cardiol. 2004. 36:405–410.27. Hung MJ, Cherng WJ, Yang NI, Cheng CW, Li LF. Relation of high-sensitivity C-reactive protein level with coronary vasospstic angina pectoris in patients without hemodynamically significant coronary artery disease. Am J Cardiol. 2005. 96:1484–1490.28. Jansson TH, Nilson TK, Johnson O. von Willebrand factor in plasma: a novel risk factor for recurrent myocardial infarction and death. Br Heart J. 1991. 66:351–355.29. Meade TW, Cooper JA, Stirling Y, Howarth DJ, Ruddock V, Miller GJ. Factor VIII, ABO blood group and the incidence of ischemic heart disease. Br J Haematol. 1994. 88:601–607.30. Synn YC, Bae JH, Kim KY. Correlation between endothelial function and the extent of coronary atherosclerosis. Korean Circ J. 2004. 34:752–760.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cardiovascular Risk Factors Predicting Endothelial Dysfunction in Patients with Variant Angina
- Effects of a Smoking Cessation Education on Smoking Cessation, Endothelial Function, and Serum Carboxyhemoglobin in Male Patients with Variant Angina
- A case of complete atrioventricular block persisting for 5 days in a patient with variant angina
- Reversed Circadian Variation in Variant Angina
- A Case of Refractory Variant Angina Relieved by Clonidine