Korean Circ J.
2008 Jan;38(1):7-11. 10.4070/kcj.2008.38.1.7.
Preclinical Test of an Electro-Mechanical Implantable Left Ventricular Assist System
- Affiliations
-
- 1Division of Thoracic and Cardiovascular Surgery, Yonsei University College of Medicine, Seoul, Korea. bcchang@yumc.yonsei.ac.kr
- 2Yonsei Cardiovascular Research Center, Yonsei University College of Medicine, Seoul, Korea.
- 3Anesthesiology, Yonsei Cardiovascular Center, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2225867
- DOI: http://doi.org/10.4070/kcj.2008.38.1.7
Abstract
- BACKGROUND AND OBJECTIVES
Ventricular assist systems are used in patients with end-stage heart failure to prolong life or as a bridge to transplantation. Several types of ventricular assist systems have been developed and they are now being used. We developed a new Biomedlab(R) electro-mechanical implantable ventricular assist device (IVAD) and we performed in vivo experimentation to evaluate the durability and safety of the device, as well as its hematologic effect.
MATERIALS AND METHODS
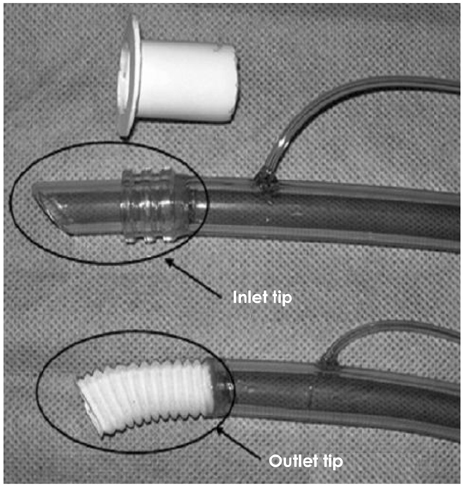
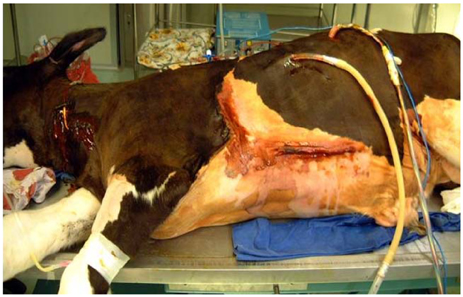
We implanted the newly developed IVAD in the pre-peritoneal cavity of 5 Hallstein calves. The inflow tract was inserted through the left ventricular apex, and the outflow tract was anastomosed to the descending thoracic aorta. Postoperatively, we administered heparin intravenously for 2 days after implantation, and then we administered warfarin sodium daily. We examined, both preoperatively and postoperatively, the serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), blood urea nitrogen (BUN), creatinine, lactate dehydrogenase (LDH), haptoglobin, fibrinogen, whole blood hemoglobin, hematocrit, prothrombin time (PT), partial thromboplastin time (PTT) and the plasma hemoglobin. We also recorded the assisted flow rate and the hemodynamic parameters of the animals. After IVAD implantation, the international normalized ratio (INR) was monitored and maintained in the range of 3.5-4.0. Postoperatively, when any device-related problems developed, we euthanized the animals and performed autopsy.
RESULTS
After IVAD implantation, the 5 calves lived for 1, 6, 3, 12 and 21 days, respectively. Three of them were euthanized due to mechanical problems such as electrical shorts, and the other calves died suddenly due to blood leakage at the outflow tract on postoperative day 21 and graft disconnection on postoperative day 3, respectively. Autopsy was performed in all the animals and there was no evidence of thromboembolism or hemorrhage in the kidney, liver or lungs. There was also no evidence of thrombosis on the valve, blood sac or inflow/outflow tract. Hematologic and chemical examinations revealed mild hemolysis in the early postoperative period, which stabilized with minimal hemolysis. There was no organ dysfunction.
CONCLUSION
Our newly developed Biomedlab(R) IVAD was feasible for implantation and it functioned well in a calf model. Although there were 3 mechanical problems, we did not find any device-related thrombosis and serious hemolysis. With this encouraging result, it may be possible to perform animal experiments with the final version of IVAD, after correcting the mechanical problems, to evaluate the device's longterm durability and stability.
MeSH Terms
-
Alanine Transaminase
Animal Experimentation
Animals
Aorta, Thoracic
Aspartate Aminotransferases
Autopsy
Blood Urea Nitrogen
Creatinine
Fibrinogen
Haptoglobins
Heart Failure
Heart Transplantation
Heart-Assist Devices
Hematocrit
Hemodynamics
Hemoglobins
Hemolysis
Hemorrhage
Heparin
Humans
International Normalized Ratio
Kidney
L-Lactate Dehydrogenase
Liver
Lung
Partial Thromboplastin Time
Plasma
Postoperative Period
Prothrombin Time
Thromboembolism
Thrombosis
Transplants
Warfarin
Alanine Transaminase
Aspartate Aminotransferases
Creatinine
Fibrinogen
Haptoglobins
Hemoglobins
Heparin
L-Lactate Dehydrogenase
Warfarin
Figure
Reference
-
1. Lee S, Park YH, Lim SH, Kwak YT, Kim H, Chang BC. Successful mechanical circulatory support as a bridge to transplantation. Asian Cardiovasc Thorac Ann. 2007. 15:243–245.2. Frazier OH, Rose EA, McCarthy P, et al. Improved mortality and rehabilitation of transplant candidates treated with a long-term implantable left ventricular assist system. Ann Surg. 1995. 222:327–338.3. Cho HS, Kim WG, Lee WY, et al. Development and evaluation of a novel electro-mechanical implantable ventricular assist system. J Biomed Eng Res. 2001. 22:349–358.4. Meuris B, Arnout J, Vlasselaers D, Schetz M, Meyns B. Long-term management of an implantable left ventricular assist device using low molecular weight heparin and antiplatelet therapy: a possible alternative to oral anticoagulants. Artif Organs. 2007. 31:402–405.5. Hetzer R, Weng Y, Potapov EV, et al. First experiences with a novel magnetically suspended axial flow left ventricular assist device. Eur J Cardiothorac Surg. 2004. 25:964–970.6. Delgado R 3rd, Frazier OH, Myers TJ, et al. Direct thrombolytic therapy for intraventricular thrombosis inpatients with the Jarvik 2000 left ventricular assist device. J Heart Lung Transplant. 2005. 24:231–233.7. Rothenburger M, Wilhelm MJ, Hammel D, et al. Treatment of thrombus formation associated with the MicroMed DeBakey VAD using recombinant tissue plasminogen activator. Circulation. 2002. 106:Suppl. I189–I192.8. Yamanaka H, Rosenberg G, Weiss WJ, Snyder AJ, Zapanta CM, Siedleki CA. Short-term in vivo studies of surface thrombosis in a left ventricular assist system. ASAIO J. 2006. 52:257–265.9. Farrar DJ, Holman WR, McBride LR, et al. Long-term follow-up of Thoratec ventricular assist device bridge-to-recovery patients successfully removed from support after recovery of ventricular function. J Heart Lung Transplant. 2002. 21:516–521.10. Frazier OH, Myers TJ, Westaby S, Gregoric ID. Use of the Jarvik 2000 left ventricular assist system as a bridge to heart transplantation or as destination therapy for patients with chronic heart failure. Ann Surg. 2003. 237:631–636.11. Frazier OH, Myers TJ. Left ventricular assist system as a bridge to myocardial recovery. Ann Thorac Surg. 1999. 68:734–741.12. Roman J, Jeevanadam V. Destination therapy with ventricular assist device. Cardiology. 2004. 101:104–110.13. Boehmer JP. Device therapy for heart failure. Am J Cardiol. 2003. 91:53D–59D.14. Hunt SA, Frazier OH. Mechanical circulatory support and cardiac transplantation. Circulation. 1998. 97:2079–2090.15. McBride LR, Naunheim KS, Fiore AC, Moroney DA, Swartz MT. Clinical experience with 111 Thoratec ventricular assist devices. Ann Thorac Surg. 1999. 67:1233–1239.16. Minami K, El-Banayosy A, Sezai A, et al. Morbidity and outcome after mechanical ventricular support using Thoratec, Novacor, and HeartMate for bridging to heart transplantation. Artif Organs. 2000. 24:421–426.17. Deng MC, Edwards LB, Hertz MI, et al. Mechanical circulatory support device database of the international society for heart and lung transplantation: second annual report, 2004. J Heart Lung Transplant. 2004. 23:1027–1034.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Treatment of Advanced Heart Failure: Beyond Medical Treatment
- The anesthetic experience of implantable left ventricular assist device insertion: a case report
- Non-Surgical Resolution of Inflow Cannula Obstruction of a Left Ventricular Assist Device: A Case Report
- Temporary Right Ventricular Assist Device Insertion via Left Thoracotomy after Left Ventricular Assist Device Implantation
- Thrombosis in the Left Ventricle after Implantable Cardioverter-Defibrillator Implantation: A Rare Cause of Systemic Thromboembolism