Korean Circ J.
2010 Dec;40(12):625-631. 10.4070/kcj.2010.40.12.625.
The Effects of Pioglitazone in Reducing Atherosclerosis Progression and Neointima Volume in Type 2 Diabetic Patients: Prospective Randomized Study With Volumetric Intravascular Ultrasonography Analysis
- Affiliations
-
- 1Department of Cardiology, Cardiovascular Center, Korea University College of Medicine, Anam Hospital, Seoul, Korea. dslmd@kumc.or.kr
- KMID: 2225160
- DOI: http://doi.org/10.4070/kcj.2010.40.12.625
Abstract
- BACKGROUND AND OBJECTIVES
Pioglitazone has been known for its anti-atherogenic effects. We compared the effects of pioglitazone in reducing atherosclerosis progression and neointima volume in type 2 diabetic patients.
SUBJECTS AND METHODS
This was a prospective, randomized single-blinded, 8-month follow-up study. Patients with significant coronary artery stenosis were randomly assigned to either pioglitazone (n=19) or placebo (n=18) following zotarolimus-eluting stent (ZES) implantation. Intravascular ultrasonography of the culprit vessel was performed from 20 mm distal and proximal to the stent at baseline. and at 8-month, and volumetric analysis was performed. Changes in inflammation markers, insulin resistance and lipid profile were compared.
RESULTS
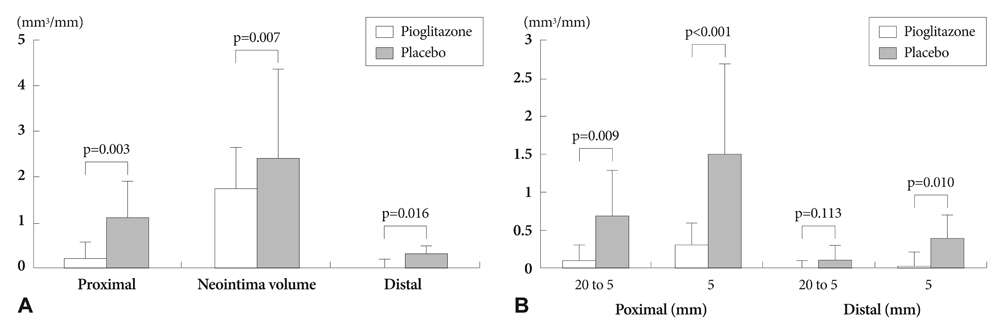
Changes in atherosclerosis progression from baseline in the pioglitazone group was significantly lower than that of the placebo group (0.06+/-0.73 vs. 1.16+/-1.41 mm3/mm, p=0.024, respectively), and neointima volume was significantly lower in the pioglitazone group compared to the placebo group (1.74+/-0.93 vs. 2.42+/-1.98 mm3/mm, p=0.007, respectively). Homeostatic model assessment-index, interleukin-6, and tumor necrosis factor-alpha levels were significantly lower in the pioglitazone group at 8 months. Adiponectin levels increased significantly only in the pioglitazone group. No significant differences in retinol binding protein-4 levels between the 2 groups were seen during the 8-month follow-up period.
CONCLUSION
Compared to placebo, pioglitazone was associated with significant reduction in atherosclerosis progression and neointima formation in type 2 diabetic patients with ZES implantation.
MeSH Terms
-
Adiponectin
Atherosclerosis
Coronary Stenosis
Diabetes Mellitus
Follow-Up Studies
Glycosaminoglycans
Humans
Inflammation
Insulin Resistance
Interleukin-6
Neointima
Prospective Studies
Stents
Thiazolidinediones
Tumor Necrosis Factor-alpha
Ultrasonography, Interventional
Vitamin A
Adiponectin
Glycosaminoglycans
Interleukin-6
Thiazolidinediones
Tumor Necrosis Factor-alpha
Vitamin A
Figure
Cited by 1 articles
-
Effects of a PPAR-γ (Peroxisome Proliferator-Activated Receptor-gamma) Activator on Flow-Mediated Brachial Artery Dilation and Circulating Level of microRNA-21 in Hypertensive Type 2 Diabetic Patients
Ji Weon Lee, Soon Jun Hong, Han Saem Jeong, Hyung Joon Joo, Jae Hyoung Park, Chul-Min Ahn, Cheol Woong Yu, Do-Sun Lim
J Korean Soc Hypertens. 2013;19(4):99-111. doi: 10.5646/jksh.2013.19.4.99.
Reference
-
1. King H, Aubert RE, Herman WH. Global burden of diabetes, 1995-2025: prevalence, numerical estimates, and projections. Diabetes Care. 1998. 21:1414–1431.2. Hong SJ, Kim MH, Ahn TH, et al. Comparison of the predictors of coronary restenosis after drug-eluting stent implantation in diabetic and nondiabetic patients. Korean Circ J. 2007. 37:530–537.3. Lee MG, Jeong MH, Ahn YK, et al. Comparison of clinical outcomes following acute myocardial infarctions in hypertensive patients with or without diabetes. Korean Circ J. 2009. 39:243–250.4. Pfützner A, Marx N, Lübben G, et al. Improvement of cardiovascular risk markers by pioglitazone is independent from glycemic control: results from the pioneer study. J Am Coll Cardiol. 2005. 45:1925–1931.5. Ceriello A. Thiazolidinediones as anti-inflammatory and anti-atherogenic agents. Diabetes Metab Res Rev. 2008. 24:14–26.6. Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007. 356:2457–2471.7. Forst T, Hohberg C, Fuellert SD, et al. Pharmacological PPARγ stimulation in contrast to beta cell stimulation results in an improvement in adipo nectin and proinsulin intact levels and reduces intima media thickness in patients with type 2 diabetes. Horm Metab Res. 2005. 37:521–527.8. Langenfeld MR, Forst T, Hohberg C, et al. Pioglitazone decreases carotid intima-media thickness independently of glycemic control in patients with type 2 diabetes mellitus: results from a controlled randomized study. Circulation. 2005. 111:2525–2531.9. Nissen SE, Nicholls SJ, Wolski K, et al. Comparison of pioglitazone vs glimepiride on progression of coronary atherosclerosis in patients with type 2 diabetes: the PERISCOPE randomized controlled trial. JAMA. 2008. 299:1561–1573.10. Tamborlane WV, Ahern J. Implications and results of the diabetes control and complications trial. Pediatr Clin North Am. 1997. 44:285–300.11. Stettler C, Allemann S, Jüni P, et al. Glycemic control and macrovascular disease in types 1 and 2 diabetes mellitus: meta-analysis of randomized trials. Am Heart J. 2006. 152:27–38.12. Tontonoz P, Hu E, Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell. 1994. 79:1147–1156.13. Wu Z, Xie Y, Morrison RF, Bucher NL, Farmer SR. PPARgamma induces the insulin-dependent glucose transporter GLUT4 in the absence of C/EBPalpha during the conversion of 3T3 fibroblasts into adipocytes. J Clin Invest. 1998. 101:22–32.14. Fonseca VA. Rationale for the use of insulin sensitizers to prevent cardiovascular events in type 2 diabetes mellitus. Am J Med. 2007. 120:9 Suppl 2. S18–S25.15. Yoon KH, Lee JH, Kim JW, et al. Epidemic obesity and type 2 diabetes in Asia. Lancet. 2006. 368:1681–1688.16. Chen KW, Boyko EJ, Bergstrom RW, et al. Earlier appearance of impaired insulin secretion than of visceral adiposity in the pathogenesis of NIDDM. 5-Year follow-up of initially nondiabetic Japanese-American men. Diabetes Care. 1995. 18:747–753.17. Matsumoto K, Miyake S, Yano M, et al. Glucose tolerance, insulin secretion, and insulin sensitivity in nonobese and obese Japanese subjects. Diabetes Care. 1997. 20:1562–1568.18. Shimizu H, Oh-I S, Tsuchiya T, Ohtani KI, Okada S, Mori M. Pioglitazone increases circulating adiponectin levels and subsequently reduces TNF-α levels in Type 2 diabetic patients: a randomized study. Diabet Med. 2006. 23:253–257.19. Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993. 259:87–91.20. Desouza CV, Murthy SN, Diez J, et al. Differential effects of peroxisome proliferator activator receptor-alpha and gamma ligands on intimal hyperplasia after balloon catheter-induced vascular injury in Zucker rats. J Cardiovasc Pharmacol Ther. 2003. 8:297–305.21. Dormandy JA, Charbonnel B, Eckland DJ, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005. 366:1279–1289.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Low Dose Pioglitazone on Restenosis and Coronary Atherosclerosis in Diabetic Patients Undergoing Drug Eluting Stent Implantation
- Effects of Pioglitazone on Cerebral Hemodynamics in Patients of Type 2 Diabetes
- Choroidal Thickening Induced by Pioglitazone in Diabetic Patients
- Multimodal intravascular photoacoustic and ultrasound imaging
- Effects of a PPAR-gamma (Peroxisome Proliferator-Activated Receptor-gamma) Activator on Flow-Mediated Brachial Artery Dilation and Circulating Level of microRNA-21 in Hypertensive Type 2 Diabetic Patients