J Rheum Dis.
2012 Aug;19(4):173-188. 10.4078/jrd.2012.19.4.173.
Animal Models in Systemic Lupus Erythematosus
- Affiliations
-
- 1Department of Internal Medicine, Eulji University College of Medicine, Eulji Medi-Bio Research Institute, Daejeon, Korea. ssc@eulji.ac.kr
- KMID: 2223069
- DOI: http://doi.org/10.4078/jrd.2012.19.4.173
Abstract
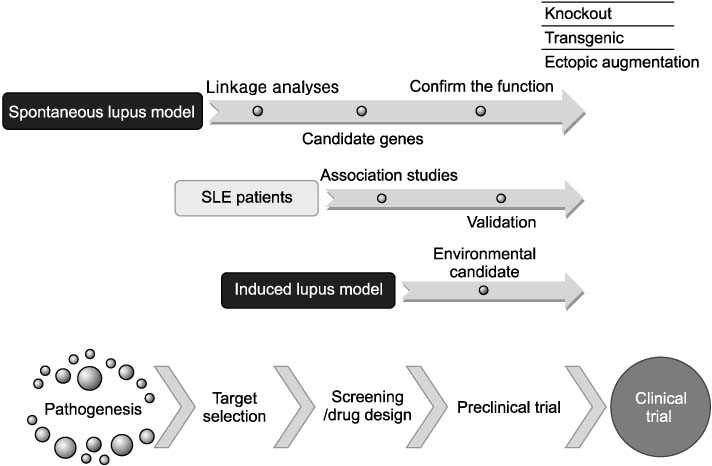
- Systemic lupus erythematosus (SLE) is an autoimmune disorder affected by multiple genetic, hormonal and environmental factors, which makes it impossible to identify the exact cause of this ailment by only investigating SLE patients, who are genetically heterogeneous, and live in various environments. Therefore, the study of mouse models of lupus has provided valuable clues to help identify, and to validate, novel molecular pathways and targets implicated in the pathogenesis of the disease. While there is no perfect model to reflect all the disease phenotypes observed in human patients, disease subsets are represented in various animal models, which allows modulation of a particular pathophysiological pathway, resulting in the possibility of dissecting its specific contribution to disease development. Spontaneous mouse models of lupus have led to identification of numerous susceptibility loci, from which several candidate genes have been found, while induced models of lupus have provided insight into the role of environmental factors, as well as a better understanding of the cellular mechanisms by which SLE develops. Animal models also allow us to screen and evaluate potential preventive and therapeutic agents. Correlation of specific pathways in animal models to subsets of human disease offers the unique possibility of more accurate preclinical predictions of efficacy for single or combinatorial therapeutic approaches in the clinic. Here, we introduce various animal models of SLE, and review current data focused on genetic factors that are associated with susceptibility or phenotypes of lupus, leading into the present understanding of the genetic basis in lupus pathogenesis.
Keyword
Figure
Reference
-
1. Morel L. Genetics of SLE: evidence from mouse models. Nat Rev Rheumatol. 2010. 6:348–357.2. Roubinian JR, Talal N, Greenspan JS, Goodman JR, Siiteri PK. Effect of castration and sex hormone treatment on survival, anti-nucleic acid antibodies, and glomerulonephritis in NZB/NZW F1 mice. J Exp Med. 1978. 147:1568–1583.3. Yin Z, Bahtiyar G, Zhang N, Liu L, Zhu P, Robert ME, et al. IL-10 regulates murine lupus. J Immunol. 2002. 169:2148–2155.4. Watanabe R, Ishiura N, Nakashima H, Kuwano Y, Okochi H, Tamaki K, et al. Regulatory B cells (B10 cells) have a suppressive role in murine lupus: CD19 and B10 cell deficiency exacerbates systemic autoimmunity. J Immunol. 2010. 184:4801–4809.5. Llorente L, Richaud-Patin Y, García-Padilla C, Claret E, Jakez-Ocampo J, Cardiel MH, et al. Clinical and biologic effects of anti-interleukin-10 monoclonal antibody administration in systemic lupus erythematosus. Arthritis Rheum. 2000. 43:1790–1800.6. Waters ST, Fu SM, Gaskin F, Deshmukh US, Sung SS, Kannapell CC, et al. NZM2328: a new mouse model of systemic lupus erythematosus with unique genetic susceptibility loci. Clin Immunol. 2001. 100:372–383.7. Vinuesa CG, Goodnow CC. Illuminating autoimmune regulators through controlled variation of the mouse genome sequence. Immunity. 2004. 20:669–679.8. Waters ST, McDuffie M, Bagavant H, Deshmukh US, Gaskin F, Jiang C, et al. Breaking tolerance to double stranded DNA, nucleosome, and other nuclear antigens is not required for the pathogenesis of lupus glomerulonephritis. J Exp Med. 2004. 199:255–264.9. Croker BP, Gilkeson G, Morel L. Genetic interactions between susceptibility loci reveal epistatic pathogenic networks in murine lupus. Genes Immun. 2003. 4:575–585.10. Kumar KR, Li L, Yan M, Bhaskarabhatla M, Mobley AB, Nguyen C, et al. Regulation of B cell tolerance by the lupus susceptibility gene Ly108. Science. 2006. 312:1665–1669.11. Morel L, Tian XH, Croker BP, Wakeland EK. Epistatic modifiers of autoimmunity in a murine model of lupus nephritis. Immunity. 1999. 11:131–139.12. Sobel ES, Satoh M, Chen Y, Wakeland EK, Morel L. The major murine systemic lupus erythematosus susceptibility locus Sle1 results in abnormal functions of both B and T cells. J Immunol. 2002. 169:2694–2700.13. Lee YH, Ji JD, Song GG. Fcgamma receptor IIB and IIIB polymorphisms and susceptibility to systemic lupus erythematosus and lupus nephritis: a meta-analysis. Lupus. 2009. 18:727–734.14. Chen Y, Perry D, Boackle SA, Sobel ES, Molina H, Croker BP, et al. Several genes contribute to the production of autoreactive B and T cells in the murine lupus susceptibility locus Sle1c. J Immunol. 2005. 175:1080–1089.15. Douglas KB, Windels DC, Zhao J, Gadeliya AV, Wu H, Kaufman KM, et al. Complement receptor 2 polymorphisms associated with systemic lupus erythematosus modulate alternative splicing. Genes Immun. 2009. 10:457–469.16. Kumar KR, Li L, Yan M, Bhaskarabhatla M, Mobley AB, Nguyen C, et al. Regulation of B cell tolerance by the lupus susceptibility gene Ly108. Science. 2006. 312:1665–1669.17. Cunninghame Graham DS, Vyse TJ, Fortin PR, Montpetit A, Cai YC, Lim S, et al. CaNIOS GenES Investigators. Association of LY9 in UK and Canadian SLE families. Genes Immun. 2008. 9:93–102.18. Liu K, Li QZ, Delgado-Vega AM, Abelson AK, Sánchez E, Kelly JA, et al. Profile Study Group. Italian Collaborative Group. German Collaborative Group. Spanish Collaborative Group. Argentinian Collaborative Group. SLEGEN Consortium. Kallikrein genes are associated with lupus and glomerular basement membrane-specific antibody-induced nephritis in mice and humans. J Clin Invest. 2009. 119:911–923.19. Kumar KR, Li L, Yan M, Bhaskarabhatla M, Mobley AB, Nguyen C, et al. Regulation of B cell tolerance by the lupus susceptibility gene Ly108. Science. 2006. 312:1665–1669.20. Taylor DK, Ito E, Thorn M, Sundar K, Tedder T, Spatz LA. Loss of tolerance of anti-dsDNA B cells in mice overexpressing CD19. Mol Immunol. 2006. 43:1776–1790.21. Zhu J, Liu X, Xie C, Yan M, Yu Y, Sobel ES, et al. T cell hyperactivity in lupus as a consequence of hyperstimulatory antigen-presenting cells. J Clin Invest. 2005. 115:1869–1878.22. Kilmon MA, Wagner NJ, Garland AL, Lin L, Aviszus K, Wysocki LJ, et al. Macrophages prevent the differentiation of autoreactive B cells by secreting CD40 ligand and interleukin-6. Blood. 2007. 110:1595–1602.23. Watson ML, Rao JK, Gilkeson GS, Ruiz P, Eicher EM, Pisetsky DS, et al. Genetic analysis of MRL-lpr mice: relationship of the Fas apoptosis gene to disease manifestations and renal disease-modifying loci. J Exp Med. 1992. 176:1645–1656.24. Chu JL, Drappa J, Parnassa A, Elkon KB. The defect in Fas mRNA expression in MRL/lpr mice is associated with insertion of the retrotransposon, ETn. J Exp Med. 1993. 178:723–730.25. Lynch DH, Watson ML, Alderson MR, Baum PR, Miller RE, Tough T, et al. The mouse Fas-ligand gene is mutated in gld mice and is part of a TNF family gene cluster. Immunity. 1994. 1:131–136.26. Shlomchik MJ, Madaio MP, Ni D, Trounstein M, Huszar D. The role of B cells in lpr/lpr-induced autoimmunity. J Exp Med. 1994. 180:1295–1306.27. Vidal S, Kono DH, Theofilopoulos AN. Loci predisposing to autoimmunity in MRL-Fas lpr and C57BL/6-Faslpr mice. J Clin Invest. 1998. 101:696–702.28. Haraldsson MK, Louis-Dit-Sully CA, Lawson BR, Sternik G, Santiago-Raber ML, Gascoigne NR, et al. The lupus-related Lmb3 locus contains a disease-suppressing Coronin-1A gene mutation. Immunity. 2008. 28:40–51.29. Shlomchik MJ, Madaio MP, Ni D, Trounstein M, Huszar D. The role of B cells in lpr/lpr-induced autoimmunity. J Exp Med. 1994. 180:1295–1306.30. Chan OT, Shlomchik MJ. Cutting edge: B cells promote CD8+ T cell activation in MRL-Fas(lpr) mice independently of MHC class I antigen presentation. J Immunol. 2000. 164:1658–1662.31. Heymann F, Meyer-Schwesinger C, Hamilton-Williams EE, Hammerich L, Panzer U, Kaden S, et al. Kidney dendritic cell activation is required for progression of renal disease in a mouse model of glomerular injury. J Clin Invest. 2009. 119:1286–1297.32. Maibaum MA, Haywood ME, Walport MJ, Morley BJ. Lupus susceptibility loci map within regions of BXSB derived from the SB/Le parental strain. Immunogenetics. 2000. 51:370–372.33. Murphy ED, Roths JB. A Y chromosome associated factor in strain BXSB producing accelerated autoimmunity and lymphoproliferation. Arthritis Rheum. 1979. 22:1188–1194.34. Merino R, Shibata T, De Kossodo S, Izui S. Differential effect of the autoimmune Yaa and lpr genes on the acceleration of lupus-like syndrome in MRL/MpJ mice. Eur J Immunol. 1989. 19:2131–2137.35. Subramanian S, Tus K, Li QZ, Wang A, Tian XH, Zhou J, et al. A Tlr7 translocation accelerates systemic autoimmunity in murine lupus. Proc Natl Acad Sci U S A. 2006. 103:9970–9975.36. Lau CM, Broughton C, Tabor AS, Akira S, Flavell RA, Mamula MJ, et al. RNA-associated autoantigens activate B cells by combined B cell antigen receptor/Toll-like receptor 7 engagement. J Exp Med. 2005. 202:1171–1177.37. Santiago-Raber ML, Kikuchi S, Borel P, Uematsu S, Akira S, Kotzin BL, et al. Evidence for genes in addition to Tlr7 in the Yaa translocation linked with acceleration of systemic lupus erythematosus. J Immunol. 2008. 181:1556–1562.38. Shen N, Fu Q, Deng Y, Qian X, Zhao J, Kaufman KM, et al. Sex-specific association of X-linked Toll-like receptor 7 (TLR7) with male systemic lupus erythematosus. Proc Natl Acad Sci U S A. 2010. 107:15838–15843.39. Merino R, Fossati L, Lacour M, Lemoine R, Higaki M, Izui S. H-2-linked control of the Yaa gene-induced acceleration of lupus-like autoimmune disease in BXSB mice. Eur J Immunol. 1992. 22:295–299.40. Haywood ME, Rogers NJ, Rose SJ, Boyle J, McDermott A, Rankin JM, et al. Dissection of BXSB lupus phenotype using mice congenic for chromosome 1 demonstrates that separate intervals direct different aspects of disease. J Immunol. 2004. 173:4277–4285.41. Muñoz LE, Lauber K, Schiller M, Manfredi AA, Herrmann M. The role of defective clearance of apoptotic cells in systemic autoimmunity. Nat Rev Rheumatol. 2010. 6:280–289.42. Baudino L, Yoshinobu K, Morito N, Santiago-Raber ML, Izui S. Role of endogenous retroviruses in murine SLE. Autoimmun Rev. 2010. 10:27–34.43. Baudino L, Yoshinobu K, Dunand-Sauthier I, Evans LH, Izui S. TLR-mediated up-regulation of serum retroviral gp70 is controlled by the Sgp loci of lupus-prone mice. J Autoimmun. 2010. 35:153–159.44. Satoh M, Kumar A, Kanwar YS, Reeves WH. Anti-nuclear antibody production and immune-complex glomerulonephritis in BALB/c mice treated with pristane. Proc Natl Acad Sci U S A. 1995. 92:10934–10938.45. Smith DL, Dong X, Du S, Oh M, Singh RR, Voskuhl RR. A female preponderance for chemically induced lupus in SJL/J mice. Clin Immunol. 2007. 122:101–107.46. Richards HB, Satoh M, Shaw M, Libert C, Poli V, Reeves WH. Interleukin 6 dependence of anti-DNA antibody production: evidence for two pathways of autoantibody formation in pristane-induced lupus. J Exp Med. 1998. 188:985–990.47. Calvani N, Satoh M, Croker BP, Reeves WH, Richards HB. Nephritogenic autoantibodies but absence of nephritis in Il-12p35-deficient mice with pristane-induced lupus. Kidney Int. 2003. 64:897–905.48. Pascual V, Farkas L, Banchereau J. Systemic lupus erythematosus: all roads lead to type I interferons. Curr Opin Immunol. 2006. 18:676–682.49. Thibault DL, Graham KL, Lee LY, Balboni I, Hertzog PJ, Utz PJ. Type I interferon receptor controls B-cell expression of nucleic acid-sensing Toll-like receptors and autoantibody production in a murine model of lupus. Arthritis Res Ther. 2009. 11:R112.50. Lee PY, Weinstein JS, Nacionales DC, Scumpia PO, Li Y, Butfiloski E, et al. A novel type IIFN-producing cell subset in murine lupus. J Immunol. 2008. 180:5101–5108.51. Lee PY, Kumagai Y, Li Y, Takeuchi O, Yoshida H, Weinstein J, et al. TLR7-dependent and FcgammaR-independent production of type I interferon in experimental mouse lupus. J Exp Med. 2008. 205:2995–3006.52. Chowdhary VR, Grande JP, Luthra HS, David CS. Characterization of haemorrhagic pulmonary capillaritis: another manifestation of Pristane-induced lupus. Rheumatology (Oxford). 2007. 46:1405–1410.53. Beech JT, Thompson SJ. Anti-tumour necrosis factor therapy ameliorates joint disease in a chronic model of inflammatory arthritis. Br J Rheumatol. 1997. 36:1129.54. Via CS. Advances in lupus stemming from the parent-into-F1 model. Trends Immunol. 2010. 31:236–245.55. Lang TJ, Nguyen P, Peach R, Gause WC, Via CS. In vivo CD86 blockade inhibits CD4+ T cell activation, whereas CD80 blockade potentiates CD8+ T cell activation and CTL effector function. J Immunol. 2002. 168:3786–3792.56. Via CS, Shustov A, Rus V, Lang T, Nguyen P, Finkelman FD. In vivo neutralization of TNF-alpha promotes humoral autoimmunity by preventing the induction of CTL. J Immunol. 2001. 167:6821–6826.57. Liu W, Szalai A, Zhao L, Liu D, Martin F, Kimberly RP, et al. Control of spontaneous B lymphocyte autoimmunity with adenovirus-encoded soluble TACI. Arthritis Rheum. 2004. 50:1884–1896.58. DiLillo DJ, Hamaguchi Y, Ueda Y, Yang K, Uchida J, Haas KM, et al. Maintenance of long-lived plasma cells and serological memory despite mature and memory B cell depletion during CD20 immunotherapy in mice. J Immunol. 2008. 180:361–371.59. Sanz I, Lee FE. B cells as therapeutic targets in SLE. Nat Rev Rheumatol. 2010. 6:326–337.60. Ramanujam M, Wang X, Huang W, Schiffer L, Grimaldi C, Akkerman A, et al. Mechanism of action of transmembrane activator and calcium modulator ligand interactor-Ig in murine systemic lupus erythematosus. J Immunol. 2004. 173:3524–3534.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Transverse Myelitis as a First Manifestation of Systemic Lupus Erythematosus
- A Case Of Systemic Lupus Erythematosus Associated With Hyperthyroidism And Severe Retinopathy
- A Case of Lupus Enteritis That Developed during the Treatment of Systemic Lupus Erythematosus
- Multiple Dermatofibromas in a woman with Systemic Lupus Erythematosus
- A Ruptured Aneurysm in a Patient with Systemic Lupus Erythematosus: Case Report