J Rheum Dis.
2015 Dec;22(6):356-365. 10.4078/jrd.2015.22.6.356.
Comparative Efficacy and Safety of Febuxostat and Allopurinol in the Treatment of Hyperuricemia: A Bayesian Network Meta-analysis
- Affiliations
-
- 1Division of Rheumatology, Department of Internal Medicine, Korea University Medical Center, Korea University College of Medicine, Seoul, Korea. lyhcgh@korea.ac.kr
- KMID: 2222809
- DOI: http://doi.org/10.4078/jrd.2015.22.6.356
Abstract
OBJECTIVE
The aim of this study was to assess the relative urate-lowering efficacy and safety of febuxostat and allopurinol in hyperuricemic patients with or without gout.
METHODS
Randomized controlled trials (RCTs) examining the efficacy and safety of febuxostat compared to allopurinol or placebo in hyperuricemic patients with/without gout were included in this Bayesian network meta-analysis.
RESULTS
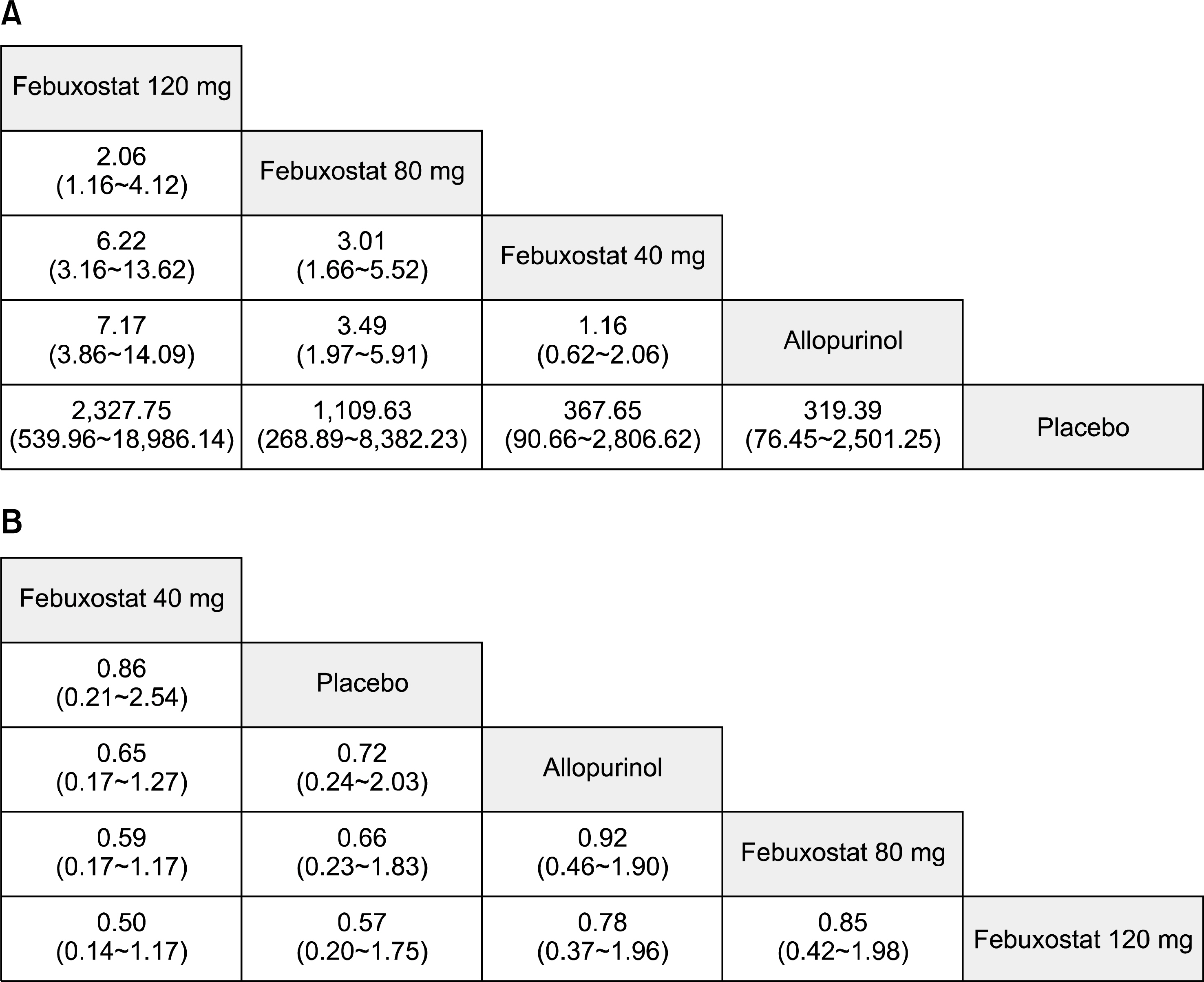
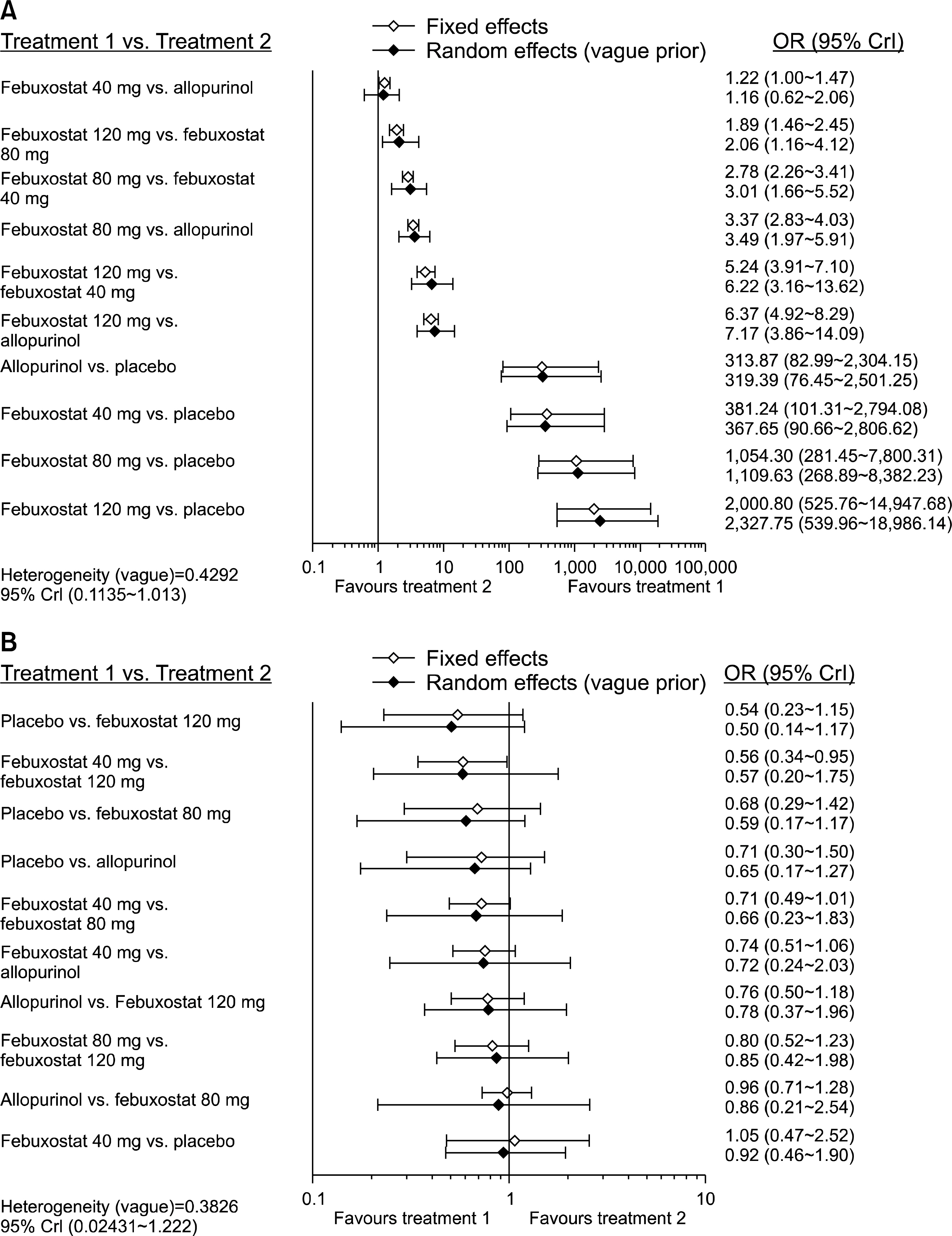
Eight RCTs including 4,099 patients met the inclusion criteria. The number of subjects achieving a serum urate (sUA) level <6.0 mg/dL was significantly higher in the febuxostat 120 mg and 80 mg groups than in the allopurinol (100 to 300 mg) group (odds ratio [OR] 7.17, 95% credible interval [CrI] 3.86 to 14.09; OR 3.49, 95% CrI 1.97 to 5.91, respectively). However, achievement of the target sUA level was comparable between febuxostat 40 mg and allopurinol. Ranking probability based on surface under the cumulative ranking curve (SUCRA) indicated that febuxostat 120 mg had the highest probability of being the best treatment for achieving the target sUA (SUCRA=0.9973), followed by febuxostat 80 mg (SUCRA=0.752), febuxostat 40 mg (SUCRA=0.4289), allopurinol (SUCRA=0.3217), and placebo (SUCRA=0). In contrast, no significant difference in safety based on the number of withdrawals due to adverse events was observed among the 5 interventions.
CONCLUSION
Febuxostat 80 mg and 120 mg were more efficacious than allopurinol (100 to 300 mg), and febuxostat 40 mg and allopurinol were comparable in urate-lowering efficacy. The safety of febuxostat at all doses was comparable with that of allopurinol.
Keyword
Figure
Cited by 1 articles
-
Rising a Novel Weapon in the War against Gout and Hyperuricemia
Jung Soo Song
J Rheum Dis. 2016;23(1):1-3. doi: 10.4078/jrd.2016.23.1.1.
Reference
-
1. Wortmann RL. Gout and hyperuricemia. Curr Opin Rheumatol. 2002; 14:281–6.
Article2. Terkeltaub RA. Clinical practice. Gout. N Engl J Med. 2003; 349:1647–55.3. Zhang W, Doherty M, Bardin T, Pascual E, Barskova V, Conaghan P, et al. EULAR Standing Committee for International Clinical Studies Including Therapeutics. EULAR evidence based recommendations for gout. Part II: management. Report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis. 2006; 65:1312–24.
Article4. Perez-Ruiz F, Lioté F. Lowering serum uric acid levels: what is the optimal target for improving clinical outcomes in gout? Arthritis Rheum. 2007; 57:1324–8.
Article5. Hande KR, Noone RM, Stone WJ. Severe allopurinol toxicity. Description and guidelines for prevention in patients with renal insufficiency. Am J Med. 1984; 76:47–56.
Article6. Arellano F, Sacristán JA. Allopurinol hypersensitivity syndrome: a review. Ann Pharmacother. 1993; 27:337–43.
Article7. Takano Y, Hase-Aoki K, Horiuchi H, Zhao L, Kasahara Y, Kondo S, et al. Selectivity of febuxostat, a novel non-purine inhibitor of xanthine oxidase/xanthine dehydrogenase. Life Sci. 2005; 76:1835–47.
Article8. Becker MA, Schumacher HR Jr, Wortmann RL, MacDonald PA, Palo WA, Eustace D, et al. Febuxostat, a novel non-purine selective inhibitor of xanthine oxidase: a twen-ty-eight-day, multicenter, phase II, randomized, doubleblind, placebo-controlled, doseresponse clinical trial examining safety and efficacy in patients with gout. Arthritis Rheum. 2005; 52:916–23.
Article9. Becker MA, Schumacher HR Jr, Wortmann RL, MacDonald PA, Eustace D, Palo WA, et al. Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N Engl J Med. 2005; 353:2450–61.
Article10. Schumacher HR Jr, Becker MA, Wortmann RL, Macdonald PA, Hunt B, Streit J, et al. Effects of febuxostat versus allopurinol and placebo in reducing serum urate in subjects with hyperuricemia and gout: a 28-week, phase III, randomized, double-blind, parallel-group trial. Arthritis Rheum. 2008; 59:1540–8.
Article11. Becker MA, Schumacher HR, Espinoza LR, Wells AF, MacDonald P, Lloyd E, et al. The urate-lowering efficacy and safety of febuxostat in the treatment of the hyperuricemia of gout: the CONFIRMS trial. Arthritis Res Ther. 2010; 12:R63.
Article12. Kamatani N, Fujimori S, Hada T, Hosoya T, Kohri K, Nakamura T, et al. An allopurinol-controlled, randomized, double-dummy, double-blind, parallel between-group, comparative study of febuxostat (TMX-67), a non-pu-rine-selective inhibitor of xanthine oxidase, in patients with hyperuricemia including those with gout in Japan: phase 3 clinical study. J Clin Rheumatol. 2011; 17(4 Suppl 2):S13–8.13. Kamatani N, Fujimori S, Hada T, Hosoya T, Kohri K, Nakamura T, et al. Placebo-controlled double-blind doseresponse study of the non-purine-selective xanthine oxidase inhibitor febuxostat (TMX-67) in patients with hyperuricemia (including gout patients) in japan: late phase 2 clinical study. J Clin Rheumatol. 2011; 17(4 Suppl 2):S35–43.14. Kamatani N, Fujimori S, Hada T, Hosoya T, Kohri K, Nakamura T, et al. Placebo-controlled, double-blind study of the non-purine-selective xanthine oxidase inhibitor Febuxostat (TMX-67) in patients with hyperuricemia including those with gout in Japan: phase 3 clinical study. J Clin Rheumatol. 2011; 17(4 Suppl 2):S19–26.15. Park SH, Song YW, Park W, Koh EM, Yoo B, Lee SK, et al. The urate-lowering efficacy and safety of febuxostat in Korean patients with gout. J Rheum Dis. 2013; 20:223–30.
Article16. Elion GB, Yü TF, Gutman AB, Hitchings GH. Renal clearance of oxipurinol, the chief metabolite of allopurinol. Am J Med. 1968; 45:69–77.
Article17. Hamburger M, Baraf HS, Adamson TC 3rd, Basile J, Bass L, Cole B, et al. European League Against Rheumatism. 2011 recommendations for the diagnosis and management of gout and hyperuricemia. Postgrad Med. 2011; 123(6 Suppl 1):3–36.
Article18. Ye P, Yang S, Zhang W, Lv Q, Cheng Q, Mei M, et al. Efficacy and tolerability of febuxostat in hyperuricemic patients with or without gout: a systematic review and meta-analysis. Clin Ther. 2013; 35:180–9.
Article19. Lee YH, Bae SC, Choi SJ, Ji JD, Song GG. Associations between vitamin D receptor polymorphisms and susceptibility to rheumatoid arthritis and systemic lupus erythematosus: a meta-analysis. Mol Biol Rep. 2011; 38:3643–51.
Article20. Lee YH, Rho YH, Choi SJ, Ji JD, Song GG. PADI4 polymorphisms and rheumatoid arthritis susceptibility: a meta-analysis. Rheumatol Int. 2007; 27:827–33.
Article21. Catalá-López F, Tobías A, Cameron C, Moher D, Hutton B. Network meta-analysis for comparing treatment effects of multiple interventions: an introduction. Rheumatol Int. 2014; 34:1489–96.
Article22. Caldwell DM, Ades AE, Higgins JP. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ. 2005; 331:897–900.
Article23. Wallace SL, Robinson H, Masi AT, Decker JL, McCarty DJ, Yü TF. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis Rheum. 1977; 20:895–900.
Article24. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996; 17:1–12.
Article25. Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and metaanalyses: the PRISMA statement. Ann Intern Med. 2009; 151:264–9.
Article26. Brown S, Hutton B, Clifford T, Coyle D, Grima D, Wells G, et al. A Microsoft-Excel-based tool for running and critically appraising network metaanalyses: an overview and application of NetMetaXL. Syst Rev. 2014; 3:110.
Article27. Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multi-ple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. 2011; 64:163–71.
Article28. Dias S, Welton NJ, Sutton AJ, Caldwell DM, Lu G, Ades AE. Evidence synthesis for decision making 4: inconsistency in networks of evidence based on randomized controlled trials. Med Decis Making. 2013; 33:641–56.29. Higgins JP, Jackson D, Barrett JK, Lu G, Ades AE, White IR. Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies. Res Synth Methods. 2012; 3:98–110.
Article30. van Valkenhoef G, Lu G, de Brock B, Hillege H, Ades AE, Welton NJ. Automating network meta-analysis. Res Synth Methods. 2012; 3:285–99.
Article31. Chohan S, Becker MA, MacDonald PA, Chefo S, Jackson RL. Women with gout: efficacy and safety of urate-lowering with febuxostat and allopurinol. Arthritis Care Res (Hoboken). 2012; 64:256–61.
Article32. Jackson RL, Hunt B, MacDonald PA. The efficacy and safety of febuxostat for urate lowering in gout patients ≥ 65 years of age. BMC Geriatr. 2012; 12:11.
Article33. Wells AF, MacDonald PA, Chefo S, Jackson RL. African American patients with gout: efficacy and safety of febuxostat vs allopurinol. BMC Musculoskelet Disord. 2012; 13:15.
Article34. Becker MA, Schumacher HR, MacDonald PA, Lloyd E, Lademacher C. Clinical efficacy and safety of successful longterm urate lowering with febuxostat or allopurinol in subjects with gout. J Rheumatol. 2009; 36:1273–82.
Article35. Kamatani N, Fujimori S, Hada T, Hosoya T, Kohri K, Nakamura T, et al. An allopurinol-controlled, multicenter, randomized, open-label, parallel between-group, comparative study of febuxostat (TMX-67), a non-purine-se-lective inhibitor of xanthine oxidase, in patients with hyperuricemia including those with gout in Japan: phase 2 exploratory clinical study. J Clin Rheumatol. 2011; 17(4 Suppl 2):S44–9.36. Schumacher HR Jr, Becker MA, Lloyd E, MacDonald PA, Lademacher C. Febuxostat in the treatment of gout: 5-yr findings of the FOCUS efficacy and safety study. Rheumatology (Oxford). 2009; 48:188–94.
Article37. Goldfarb DS, MacDonald PA, Hunt B, Gunawardhana L. Febuxostat in gout: serum urate response in uric acid over-producers and underexcretors. J Rheumatol. 2011; 38:1385–9.
Article38. Sezai A, Soma M, Nakata K, Hata M, Yoshitake I, Wakui S, et al. Comparison of febuxostat and allopurinol for hyperuricemia in cardiac surgery patients (NU-FLASH Trial). Circ J. 2013; 77:2043–9.
Article39. White WB, Chohan S, Dabholkar A, Hunt B, Jackson R. Cardiovascular safety of febuxostat and allopurinol in patients with gout and cardiovascular comorbidities. Am Heart J. 2012; 164:14–20.
Article40. Perez-Ruiz F, Calabozo M, Pijoan JI, Herrero-Beites AM, Ruibal A. Effect of urate-lowering therapy on the velocity of size reduction of tophi in chronic gout. Arthritis Rheum. 2002; 47:356–60.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Overview of Network Meta-analysis for a Rheumatologist
- Renoprotective effects of febuxostat compared with allopurinol in patients with hyperuricemia: A systematic review and meta-analysis
- The potential renoprotection of xanthine oxidase inhibitors: Febuxostat versus allopurinol
- Febuxostat for the Treatment of Chronic Tophaceous Gout in a Patient on Continuous Ambulatory Peritoneal Dialysis
- What is the Best Choice for Urate-lowering Therapy for Korean?