Korean Diabetes J.
2010 Jun;34(3):174-181. 10.4093/kdj.2010.34.3.174.
Is A1C Variability an Independent Predictor for the Progression of Atherosclerosis in Type 2 Diabetic Patients?
- Affiliations
-
- 1Division of Endocrinology and Metabolism, Department of Internal Medicine, Hallym University Sacred Heart Hospital, Hallym University College of Medicine, Anyang, Korea. ironeat@hallym.ac.kr
- KMID: 2222373
- DOI: http://doi.org/10.4093/kdj.2010.34.3.174
Abstract
- BACKGROUND
Little is known about the relative contribution of long-term glycemic variability to the risk of macrovascular complications in type 2 diabetes. This study was conducted to evaluate the effect of A1C variability on the progression of carotid artery intima-media thickness (IMT) in type 2 diabetic patients.
METHODS
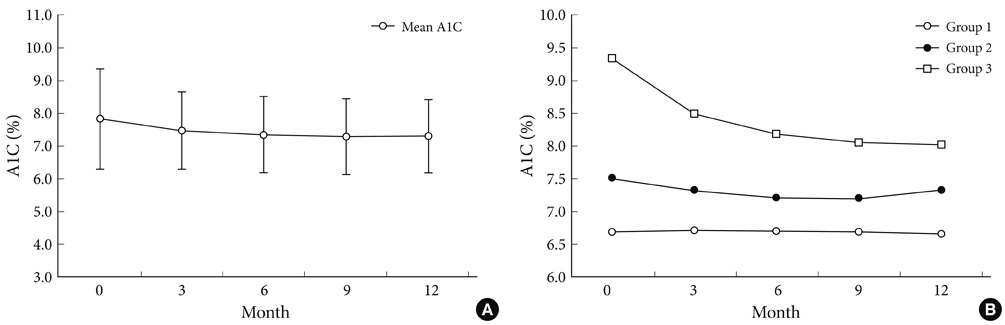
Among type 2 diabetic patients who visited Hallym University Sacred Heart Hospital from March 2007 to September 2009, 120 patients who had carotid artery IMT measured annually and A1C checked every three months for at least one year were analyzed. Individual A1C variability was defined as the standard deviation (SD) of five A1C levels taken every three months for approximately one year. Change in IMT was defined as an increase in IMT on follow-up measurement. The association between the SD of A1C and changes in IMT was evaluated.
RESULTS
With greater A1C variability, there was a greater increase in the mean IMT (r = 0.350, P < 0.001) of the carotid artery. After adjusting for confounding factors that may influence IMT, A1C variability was significantly associated with the progression of IMT (r = 0.222, P = 0.034). However, the SD of A1C was not a significant independent risk factor for the progression of IMT in multiple regression analysis (beta = 0.158, P = 0.093).
CONCLUSION
Higher A1C variability is associated with IMT progression in type 2 diabetic patients; however, it is not an independent predictor of IMT progression. Overall glycemic control is the most important factor in the progression of IMT.
MeSH Terms
Figure
Cited by 1 articles
-
HbA1c Variability and Micro- and Macrovascular Complications of Diabetes
Hae Kyung Yang, Seung-Hwan Lee
J Korean Diabetes. 2014;15(4):202-205. doi: 10.4093/jkd.2014.15.4.202.
Reference
-
1. Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000. 321:405–412.2. Laakso M, Lehto S. Epidemiology of macrovascular disease in diabetes. Diabetes Rev. 1997. 5:294–315.3. The relationship of glycemic exposure (HbA1c) to the risk of development and progression of retinopathy in the diabetes control and complications trial. Diabetes. 1995. 44:968–983.4. Monnier L, Colette C. Glycemic variability: should we and can we prevent it? Diabetes Care. 2008. 31:Suppl 2. S150–S154.5. Kilpatrick ES, Rigby AS, Atkin SL. A1C variability and the risk of microvascular complications in type 1 diabetes: data from the Diabetes Control and Complications Trial. Diabetes Care. 2008. 31:2198–2202.6. Prince CT, Becker DJ, Costacou T, Miller RG, Orchard TJ. Changes in glycaemic control and risk of coronary artery disease in type 1 diabetes mellitus: findings from the Pittsburgh Epidemiology of Diabetes Complications Study (EDC). Diabetologia. 2007. 50:2280–2288.7. Home P. Contributions of basal and post-prandial hyperglycaemia to micro- and macrovascular complications in people with type 2 diabetes. Curr Med Res Opin. 2005. 21:989–998.8. Ceriello A. Postprandial hyperglycemia and diabetes complications: is it time to treat? Diabetes. 2005. 54:1–7.9. Esposito K, Giugliano D, Nappo F, Marfella R. Regression of carotid atherosclerosis by control of postprandial hyperglycemia in type 2 diabetes mellitus. Circulation. 2004. 110:214–219.10. Hulthe J, Wikstrand J, Emanuelsson H, Wiklund O, de Feyter PJ, Wendelhag I. Atherosclerotic changes in the carotid artery bulb as measured by B-mode ultrasound are associated with the extent of coronary atherosclerosis. Stroke. 1997. 28:1189–1194.11. Hu Y, Liu W, Huang R, Zhang X. Postchallenge plasma glucose excursions, carotid intima-media thickness, and risk factors for atherosclerosis in Chinese population with type 2 diabetes. Atherosclerosis. 2010. 210:302–306.12. Butt MU, Zakaria M. Association of common carotid intimal medial thickness (CCA-IMT) with risk factors of atherosclerosis in patients with type 2 diabetes mellitus. J Pak Med Assoc. 2009. 59:590–593.13. Ho HC, Chen MF, Hwang JJ, Lee YT, Su TC. Intima-media thickness of lower-limb arteries associated with fasting and post-challenge plasma glucose levels. J Atheroscler Thromb. 2009. 16:748–755.14. Touboul PJ, Elbaz A, Koller C, Lucas C, Adrai V, Chedru F, Amarenco P. The GENIC Investigators. Common carotid artery intima-media thickness and brain infarction: the Etude du Profil Génétique de l'Infarctus Cérébral (GENIC) case-control study. Circulation. 2000. 102:313–318.15. Yokoyama H, Katakami N, Yamasaki Y. Recent advances of intervention to inhibit progression of carotid intima-media thickness in patients with type 2 diabetes mellitus. Stroke. 2006. 37:2420–2427.16. Park SW, Kim SK, Cho YW, Kim DJ, Song YD, Choi YJ, Huh BW, Choi SH, Jee SH, Cho MA, Lee EJ, Huh KB. Insulin resistance and carotid atherosclerosis in patients with type 2 diabetes. Atherosclerosis. 2009. 205:309–313.17. Kilpatrick ES, Rigby AS, Atkin SL. The effect of glucose variability on the risk of microvascular complications in type 1 diabetes. Diabetes Care. 2006. 29:1486–1490.18. Muggeo M, Verlato G, Bonora E, Zoppini G, Corbellini M, de Marco R. Long-term instability of fasting plasma glucose, a novel predictor of cardiovascular mortality in elderly patients with non-insulin-dependent diabetes mellitus: the Verona Diabetes Study. Circulation. 1997. 96:1750–1754.19. Muggeo M, Zoppini G, Bonora E, Brun E, Bonadonna RC, Moghetti P, Verlato G. Fasting plasma glucose variability predicts 10-year survival of type 2 diabetic patients: the Verona Diabetes Study. Diabetes Care. 2000. 23:45–50.20. Frier BM. Hypoglycaemia in the diabetic adult. Baillieres Clin Endocrinol Metab. 1993. 7:757–777.21. DeFronzo RA, Ferrannini E. Insulin resistance: a multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care. 1991. 14:173–194.22. Muis MJ, Bots ML, Grobbee DE, Stolk RP. Insulin treatment and cardiovascular disease; friend or foe? A point of view. Diabet Med. 2005. 22:118–126.23. Davi G, Ciabattoni G, Consoli A, Mezzetti A, Falco A, Santarone S, Pennese E, Vitacolonna E, Bucciarelli T, Costantini F, Capani F, Patrono C. In vivo formation of 8-iso-prostaglandin f2alpha and platelet activation in diabetes mellitus: effects of improved metabolic control and vitamin e supplementation. Circulation. 1999. 99:224–229.24. Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, Raskin P, Zinman B. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005. 353:2643–2653.25. Donahue RP, Abbott RD, Reed DM, Yano K. Postchallenge glucose concentration and coronary heart disease in men of Japanese ancestry. Honolulu Heart Program. Diabetes. 1987. 36:689–692.26. Coutinho M, Gerstein HC, Wang Y, Yusuf S. The relationship between glucose and incident cardiovascular events: a metaregression analysis of published data from 20 studies of 95,783 individuals followed for 12.4 years. Diabetes Care. 1999. 22:233–240.27. Bunn HF, Gabbay KH, Gallop PM. The glycosylation of hemoglobin: relevance to diabetes mellitus. Science. 1978. 200:21–27.28. Gonen B, Rubenstein A, Rochman H, Tanega SP, Horwitz DL. Haemoglobin A1: an indicator of the metabolic control of diabetic patients. Lancet. 1977. 2:734–737.29. Derr R, Garrett E, Stacy GA, Saudek CD. Is HbA(1c) affected by glycemic instability? Diabetes Care. 2003. 26:2728–2733.30. Hirsch IB, Brownlee M. Should minimal blood glucose variability become the gold standard of glycemic control? J Diabetes Complications. 2005. 19:178–181.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A1c Variability Can Predict Coronary Artery Disease in Patients with Type 2 Diabetes with Mean A1c Levels Greater than 7
- Impact of Serum Adiponectin Concentration on Progression of Carotid Atherosclerosis in Patients with Type 2 Diabetes Mellitus
- Increased Carotid Intima-Media Thickness is Associated with Progression of Diabetic Nephropathy in Patients with Type 2 Diabetes
- Relationship between Glycemic Control and Diabetic Retinopathy
- Factors Associated with Diabetic Complication Index among Type 2 Diabetes Patients: Focusing on Regular Outpatient Follow-up and HbA1c Variability