J Korean Ophthalmol Soc.
2013 Mar;54(3):496-501. 10.3341/jkos.2013.54.3.496.
Effect of Dipyridamole on the Reactive Oxygen Species and Oxidative Stress in Trabecular Meshwork Cells
- Affiliations
-
- 1Department of Ophthalmology, Catholic University of Daegu School of Medicine, Daegu, Korea. jwkim@cu.ac.kr
- KMID: 2216772
- DOI: http://doi.org/10.3341/jkos.2013.54.3.496
Abstract
- PURPOSE
To investigate the effects of dipyridamole (DPD) on the production of reactive oxygen species (ROS) and oxidative stress in cultured human trabecular meshwork cells (HTMC).
METHODS
Antioxidant activity of DPD was determined by DPPH assay. Primarily cultured HTMC were exposed to 0, 20, and 50 microm DPD using serum-deprived media. The effect of DPD on the production of ROS was assessed with the DCHFDA assay. The effect of DPD on the t-butyl hydroperoxide (tBHP)-induced oxidative stress was assessed with resazurin assay.
RESULTS
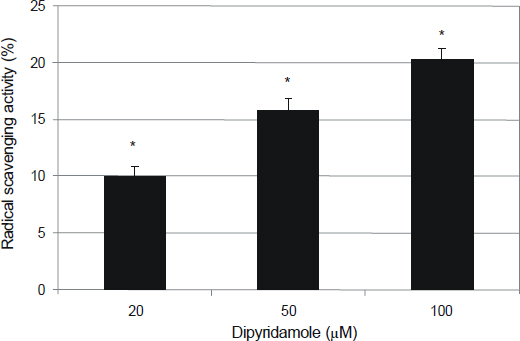
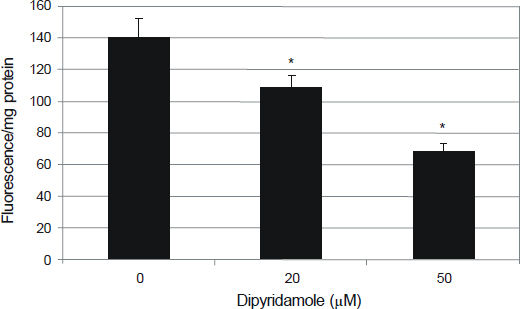
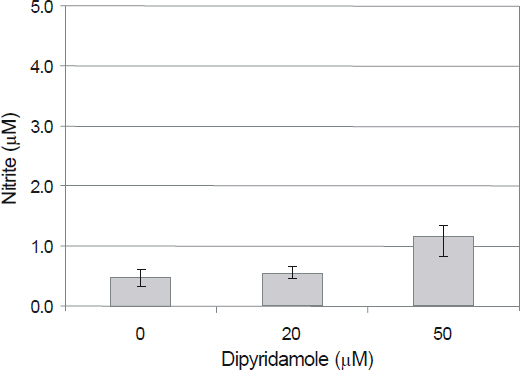
DPD showed significant antioxidant activity. DPD significantly decreased the production of ROS (p < 0.05) and improved cellular activity significantly after treatment with t-BHP (p < 0.05). DPD did not affect the generation of nitric oxides.
CONCLUSIONS
DPD suppressed the formation of ROS and possessed cytoprotective activity against the oxidative stress in HTMC.
MeSH Terms
Figure
Reference
-
References
1. Alvarado J, Murphy C, Juster R. Trabecular meshwork cellularity in primary open-angle glaucoma and nonglaucomatous normals. Ophthalmology. 1984; 91:564–79.
Article2. Rohen JW, LÜtjen-Drecoll E, FlÜgel C, et al. Ultrastructure of the trabecular meshwork in untreated cases of primary open-angle glaucoma (POAG). Exp Eye Res. 1993; 56:683–92.3. Wiederholt M, Dörschner N, Groth J. Effect of diuretics, channel modulators and signal interceptors on contractility of the trabecular meshwork. Ophthalmologica. 1997; 211:153–60.
Article4. Wiederholt M, Stumpff F. The trabecular meshwork and aqueous humor reabsorption. Civan MM, editor. Current topics in membranes. The eye's aqueous Humor: from secretion to glaucoma. v. 45. San Diego: Academic Press;1998. p. 163–202.5. Wiederholt M, Sturm A, Lepple-Wienhues A. Relaxation of trabecular meshwork and ciliary muscle by release of nitric oxide. Invest Ophthalmol Vis Sci. 1994; 35:2515–20.6. Behar-Cohen FF, Goureau O, D'Hermies F, Courtois Y. Decreased intraocular pressure induced by nitric oxide donors is correlated to nitrite production in the rabbit eye. Invest Ophthalmol Vis Sci. 1996; 37:1711–5.7. Saccà SC, Izzotti A, Rossi P, Traverso C. Glaucomatous outflow pathway and oxidative stress. Exp Eye Res. 2007; 84:389–99.
Article8. Klein JA, Ackerman SL. Oxidative stress, cell cycle, and neuro-degeneration. J Clin Invest. 2003; 111:785–93.
Article9. Ischiropoulos H, Beckman JS. Oxidative stress and nitration in neurodegeneration: cause, effect, or association? J Clin Invest. 2003; 111:163–9.
Article10. Hong JH, Kim YY, Kim JW. Effect of genistein on the survival and production of nitric oxide in trabecular meshwork cells. J Korean Ophthalmol Soc. 2011; 52:970–4.
Article11. Lee SH, Kim JW. Effect of erythropoietin on the production of nitric oxide in trabecular meshwork cells. J Korean Ophthalmol Soc. 2011; 52:1514–8.
Article12. Chakrabarti S, Vitseva O, Iyu D, et al. The effect of dipyridamole on vascular cell-derived reactive oxygen species. J Pharmacol Exp Ther. 2005; 315:494–500.
Article13. Iuliano L, Ghiselli A, Alessandri C, et al. Superoxide anion scavenging property of dipyridamole. Thromb Haemost. 1989; 61:149.
Article14. Iuliano L, Piccheri C, Coppola I, et al. Fluorescence quenching of dipyridamole associated to peroxyl radical scavenging: a versatile probe to measure the chain breaking antioxidant activity of biomolecules. Biochim Biophys Acta. 2000; 1474:177–82.
Article15. Liu F, Ng TB. Antioxidative and free radical scavenging activities of selected medicinal herbs. Life Sci. 2000; 66:725–35.
Article16. Lee SE, Hwang HJ, Ha JS, et al. Screening of medicinal plant extracts for antioxidant activity. Life Sci. 2003; 73:167–79.
Article17. Parejo I, Viladomat F, Bastida J, et al. Investigation of Bolivian plant extracts for their radical scavenging activity and antioxidant activity. Life Sci. 2003; 73:1667–81.
Article18. Wang H, Joseph JA. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic Biol Med. 1999; 27:612–6.19. Abu-Amero KK, Bosley TM. Detection of mitochondrial respiratory dysfunction in circulating lymphocytes using resazurin. Arch Pathol Lab Med. 2005; 129:1295–8.
Article20. Green LC, Wagner DA, Glogowski J, et al. Analysis of nitrate, nitrite and [15N]nitrate in biological fluids. Anal Biochem. 1982; 126:131–8.
Article21. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983; 65:55–63.
Article22. Freimoser FM, Jakob CA, Aebi M, Tuor U. The MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay is a fast and reliable method for colorimetric determination of fungal cell densities. Appl Environ Microbiol. 1999; 65:3727–9.
Article23. Polansky JR, Weinreb RN, Baxter JD, Alvarado J. Human trabecular cells. I. Establishment in tissue culture and growth characteristics. Invest Ophthalmol Vis Sci. 1979; 18:1043–9.24. Alvarado JA, Wood I, Polansky JR. Human trabecular cells. II. Growth pattern and ultrastructural characteristics. Invest Ophthalmol Vis Sci. 1982; 23:464–78.25. Saccà SC, Pascotto A, Camicione P, et al. Oxidative DNA damage in the human trabecular meshwork: clinical correlation in patients with primary open-angle glaucoma. Arch Ophthalmol. 2005; 123:458–63.26. El-Remessy AB, Abou-Mohamed G, Caldwell RW, Caldwell RB. High glucose-induced tyrosine nitration in endothelial cells: role of eNOS uncoupling and aldose reductase activation. Invest Ophthalmol Vis Sci. 2003; 44:3135–43.
Article27. Iuliano L, Colavita AR, Camastra C, et al. Protection of low density lipoprotein oxidation at chemical and cellular level by the anti-oxidant drug dipyridamole. Br J Pharmacol. 1996; 119:1438–46.
Article28. Iuliano L, Piccheri C, Coppola I, et al. Fluorescence quenching of dipyridamole associated to peroxyl radical scavenging: a versatile probe to measure the chain breaking antioxidant activity of biomolecules. Biochim Biophys Acta. 2000; 1474:177–82.
Article29. Selley ML, Czeti AL, McGuiness JA, Ardlie NG. Dipyridamole inhibits the oxidative modification of low density lipoprotein. Atherosclerosis. 1994; 111:91–7.
Article30. Farinelli SE, Greene LA, Friedman WJ. Neuroprotective actions of dipyridamole on cultured CNS neurons. J Neurosci. 1998; 18:5112–23.
Article31. Gamboa A, Abraham R, Diedrich A, et al. Role of adenosine and nitric oxide on the mechanisms of action of dipyridamole. Stroke. 2005; 36:2170–5.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Methylglyoxal on the Oxidative Stress in Trabecular Meshwork Cells
- Effect of High Glucose on the Production of Reactive Oxygen Species in Trabecular Meshwork Cells
- Cytoprotective Effect of Rho Kinase Inhibitors against Oxidative Stress inTrabecular Meshwork Cells
- Effect of Advanced Glycation End Products on Oxidative Stress and Senescence of Trabecular Meshwork Cells
- Effect of Hydrogen Peroxide-induced Oxidative Stress on the Senescence of Trabecular Meshwork Cells