J Korean Ophthalmol Soc.
2013 Feb;54(2):215-223. 10.3341/jkos.2013.54.2.215.
Efficacy and Safety of Topical Unpreserved 0.1% Fluorometholone Ophthalmic Solution on Dry Eye Syndrome
- Affiliations
-
- 1Department of Ophthalmology, Kyungpook National University School of Medicine, Daegu, Korea. okeye@knu.ac.kr
- 2Cheil Eye Hospital, Daegu, Korea.
- KMID: 2216655
- DOI: http://doi.org/10.3341/jkos.2013.54.2.215
Abstract
- PURPOSE
To evaluate the efficacy and safety of topical unpreserved 0.1% fluorometholone (FML) ophthalmic solution in patients with dry eye syndrome.
METHODS
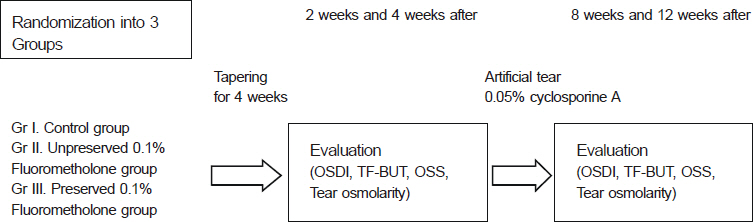
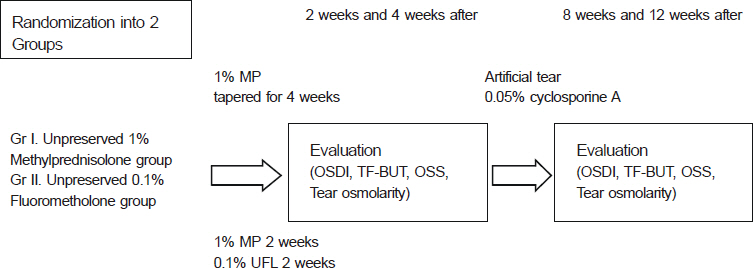
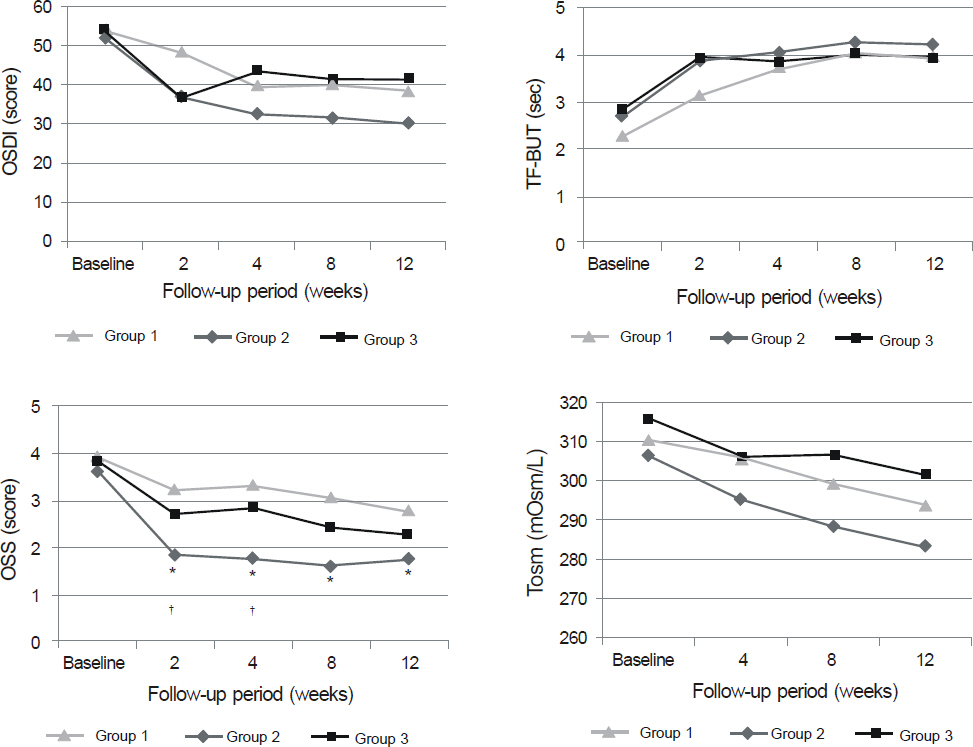
Patients with mild to moderate dry eye syndrome were divided into the control group (Group I), topical unpreserved 0.1% FML group (Group II), and topical preserved 0.1% FML group (Group III). Intraocular pressure (IOP), Ocular Surface Disease Index (OSDI), tear film break-up time (TF-BUT), Oxford stain score (OSS), and tear osmolarity (Tosm) were evaluated at 2 weeks, 4 weeks, 8 weeks, and 12 weeks (Trial 1). Patients with severe dry eye syndrome were divided into 1% methylprednisolone (MP) group (Group I) and 0.1% unpreserved FML group (Group II). Same parameters were evaluated in both groups (Trial 2).
RESULTS
In clinical trial I, OSS scores of Group II were lower than other groups (p < 0.05). For severe dry eye patients in clinical trial 2, there were no significant differences in all parameters between the 2 groups.
CONCLUSIONS
Topical unpreserved 0.1% fluorometholone was shown to be an effective and relatively safe treatment in patients with dry eye syndrome.
MeSH Terms
Figure
Reference
-
References
1. Brewitt H, Sistani F. Dry eye disease: the scale of the problem. Surv Ophthalmol. 2001; 45:199–202.2. Pflugfelder SC, Tseng SC, Sanabria O, et al. Evaluation of subjective assessments and objective diagnostic tests for diagnosing tear-film disorders known to cause ocular irritation. Cornea. 1998; 17:38–56.
Article3. Stern ME, Beuerman RW, Fox RI, et al. The pathology of dry eye: the interaction between the ocular surface and lacrimal glands. Cornea. 1998; 17:584–9.4. Pflugfelder SC. Antiinflammatory therapy for dry eye. Am J Ophthalmol. 2004; 137:337–42.
Article5. Dana M, Hamrah P. Role of immunity and inflammation in corneal and ocular surface disease associated with dry eye. Adv Exp Med Biol. 2002; 506:729–38.
Article6. Barton K, Monroy DC, Nava A, Pflugfelder SC. Inflammatory cytokines in the tears of patients with ocular rosacea. Ophthalmology. 1997; 104:1868–74.
Article7. Pflugfelder SC, Jones D, Ji Z, et al. Altered cytokine balance in the tear fluid and conjunctiva of patients with Sjögren's syndrome keratoconjunctivitis sicca. Curr Eye Res. 1999; 19:201–11.
Article8. Tishler M, Yaron I, Geyer O, et al. Elevated tear interleukin-6 levels in patients with Sjögren syndrome. Ophthalmology. 1998; 105:2327–9.
Article9. Yoon KC, Jeong IY, Park YG, Yang SY. Interleukin-6 and tumor necrosis factor-alpha levels in tears of patients with dry eye syndrome. Cornea. 2007; 26:431–7.10. Stevenson D, Tauber J, Reis BL. Efficacy and safety of cyclosporin A ophthalmic emulsion in the treatment of moderate-to-severe dry eye disease: a dose-ranging, randomized trial. The Cyclosporin A Phase 2 Study Group. Ophthalmology. 2000; 107:967–74.11. Power WJ, Mullaney P, Farrell M, Collum LM. Effect of topical cyclosporin A on conjunctival T cells in patients with secondary Sjögren's syndrome. Cornea. 1993; 12:507–11.
Article12. Borel JF, Baumann G, Chapman I, et al. In vivo Pharmacological effects of ciclosporin and some analogues. Adv Pharmacol. 1996; 35:115–246.
Article13. Marsh P, Pflugfelder SC. Topical nonpreserved methylprednisolone therapy for keratoconjunctivitis sicca in Sjögren's syndrome. Ophthalmology. 1999; 106:811–6.14. Hong S, Kim T, Chung SH, et al. Recurrence after topical non-preserved methylprednisolone therapy for keratoconjunctivitis sicca in Sjögren's syndrome. J Ocul Pharmacol Ther. 2007; 23:78–82.
Article15. Avunduk AM, Avunduk MC, Varnell ED, Kaufman HE. The comparison of efficacies of topical corticosteroids and nonsteroidal anti-inflammatory drops on dry eye patients: a clinical and immunocytochemical study. Am J Ophthalmol. 2003; 136:593–602.
Article16. Burstein NL. Preservative cytotoxic threshold for benzalkonium chloride and chlorhexidine digluconate in cat and rabbit corneas. Invest Ophthalmol Vis Sci. 1980; 19:308–13.17. Burstein NL. Corneal cytotoxicity of topically applied drugs, vehicles and preservatives. Surv Ophthalmol. 1980; 25:15–30.
Article18. Wilson SE, Stulting RD. Agreement of physician treatment practices with the international task force guidelines for diagnosis and treatment of dry eye disease. Cornea. 2007; 26:284–9.
Article19. The definition and classification of dry eye disease: repot of the Definition and Classification Subcommittee of the International Dry Eye Workshop (2007). Ocul Surf. 2007; 5:75–92.20. Kim WJ, Kim HS, Kim MS. Current trends in the recognition and treatment of dry eye: a survey of ophthalmologists. J Korean Ophthalmol Soc. 2007; 48:1614–22.
Article21. Lee JS, Jung DY, Oum BS, Kim CD. Cytotoxicity of benzalkonium chloride on the corneal epithelial cell of rabbit. J Korean Ophthalmol Soc. 1998; 39:1326–33.22. Epstein SP, Chen D, Asbell PA. Evaluation of biomarkers of inflammation in response to benzalkonium chloride on corneal and conjunctival epithelial cells. J Ocul Pharmacol Ther. 2009; 25:415–24.
Article23. Epstein SP, Ahdoot M, Marcus E, Asbell PA. Comparative toxicity of preservatives on immortalized corneal and conjunctival epithelial cells. J Ocul Pharmacol Ther. 2009; 25:113–9.
Article24. Lee JK, Ryu YH. The effect of antiglaucoma medication on cultured human conjunctival epithelial cells. J Korean Ophthalmol Soc. 2006; 47:1811–8.25. Pisella PJ, Debbasch C, Hamard P, et al. Conjunctival proinflammatory and proapoptotic effects of latanoprost and preserved and unpreserved timolol: an ex vivo and in vitro study. Invest Ophthalmol Vis Sci. 2004; 45:1360–8.
Article26. de Jong C, Stolwijk T, Kuppens E, et al. Topical timolol with and without benzalkonium chloride: epithelial permeability and autofluorescence of the cornea in glaucoma. Graefes Arch Clin Exp Ophthalmol. 1994; 232:221–4.
Article27. De Saint Jean M, Brignole F, Bringuier AF, et al. Effects of benzalkonium chloride on growth and survival of Chang conjunctival cells. Invest Ophthalmol Vis Sci. 1999; 40:619–30.28. Jaenen N, Baudouin C, Pouliquen P, et al. Ocular symptoms and signs with preserved and preservative-free glaucoma medications. Eur J Ophthalmol. 2007; 17:341–9.
Article29. Marsh P, Pflugfelder SC. Topical nonpreserved methylprednisolone therapy for keratoconjunctivitis sicca in Sjögren syndrome. Ophthalmology. 1999; 106:811–6.
Article30. Albert DM, Miller JW, Azar DT, et al. Albert & Jakobiec's principles and practice of ophthalmology. 3rd ed. v. 1. Philadelphia: Elsevier;2008. p. 249–58.31. Khanal S, Millar TJ. Barriers to clinical uptake of tear osmolarity measurements. Br J Ophthalmol. 2012; 96:341–4.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Points to consider when developing drugs for dry eye syndrome
- Effectiveness of Cyclosporine-steroid Treatment after Cataract Surgery according to Dry Eye Severity
- Long-Term Outcome of Treatment with Topical Corticosteroids for Severe Dry Eye Associated with Sjogren's Syndrome
- An Automatic System for the Delivery of Eye-Drops Using a Microinfusion Pump
- Clinical Efficacy of Topical 3% Diquafosol Tetrasodium in Short Tear Film Break-Up Time Dry Eye