J Korean Ophthalmol Soc.
2011 Jul;52(7):816-824. 10.3341/jkos.2011.52.7.816.
Combined Photodynamic Therapy and Intravitreal Bevacizumab Injection for Exudative Age-Related Macular Degeneration and Polypoidal Choroidal Vasculopathy
- Affiliations
-
- 1Department of Ophthalmology, Pusan National University School of Medicine, Busan, Korea. jlee@pusan.ac.kr
- KMID: 2214706
- DOI: http://doi.org/10.3341/jkos.2011.52.7.816
Abstract
- PURPOSE
To compare the efficacies of photodynamic therapy (PDT) using verteporfin combined with intravitreal bevacizumab in choroidal neovascularization secondary to age-related macular degeneration and polypoidal choroidal vasculopathy (PCV).
METHODS
Photodynamic therapy followed by 3 monthly intravitreal injections of bevacizumab were performed for neovascular age-related macular degeneration (AMD group) and PCV (PCV group). The injections were performed within 2 weeks after PDT and then every 4 to 6 weeks. During the 12-month follow-up period, best-corrected visual acuity (BCVA), central subfield macular thickness (CSMT), and number of reinjections were reviewed retrospectively.
RESULTS
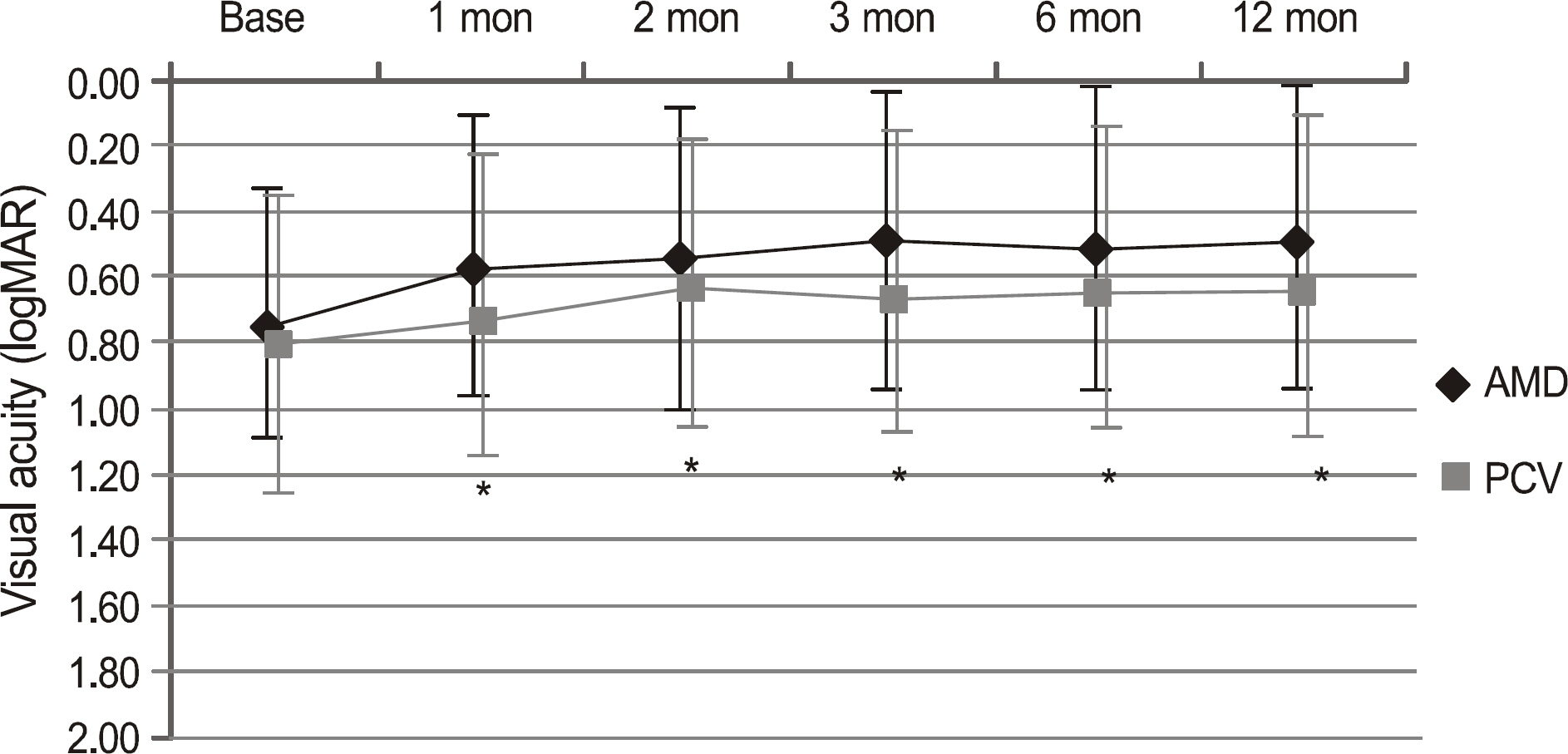
Out of 38 eyes, there were 21 eyes in the AMD group (mean age 66.8 years old) and 17 eyes in the PCV group (mean age 66.4 years old). The average follow-up duration was 17.0 months. At 12 months, the BCVA (logMAR) improved from 0.75 at baseline to 0.49 in the AMD group (p = 0.01) and from 0.81 to 0.63 in the PCV group (p = 0.03). The CSMT decreased significantly from 329 to 231 in the AMD group and from 354 to 223 in the PCV group. At 12 months, 20 eyes (95.2%) in the AMD group and 15 eyes (88.2%) in the PCV group increased or maintained BCVA. The numbers of reinjections were 4.3 in the AMD group and 3.0 in the PCV group. There were no significant differences in BCVA, BCVA changes, CSMT, or number of reinjection between the two groups.
CONCLUSIONS
Combined PDT and intravitreal bevacizumab injections showed no significant difference in stabilization of vision or retreatment rates between neovascular age-related macular degeneration and PCV.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Friedman DS, O'Colmain BJ, Muñoz B, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004; 122:564–72.
Article2. Klein R, Klein BE, Knudtson MD, et al. Prevalence of age-related macular degeneration in 4 racial/ethnic groups in the multi-ethnic study of atherosclerosis. Ophthalmology. 2006; 113:373–80.
Article3. Yannuzzi LA, Sorenson J, Spaide RF, Lipson B. Idiopathic polypoidal choroidal vasculopathy (IPCV). Retina. 1990; 10:1–8.
Article4. Spaide RF, Yannuzzi LA, Slakter JS, et al. Indocyanine green videoangiography of idiopathic polypoidal choroidal vasculopathy. Retina. 1995; 15:100–10.
Article5. Yannuzzi LA, Ciardella A, Spaide RF, et al. The expanding clinical spectrum of idiopathic polypoidal choroidal vasculopathy. Arch Ophthalmol. 1997; 115:478–85.
Article6. Lee WK, Kwon SI. Polypoidal choroidal vasculopathy. J Korean Ophthalmol Soc. 2000; 41:2573–84.7. Uyama M, Wada M, Nagai Y, et al. Polypoidal choroidal vasculopathy: natural history. Am J Ophthalmol. 2002; 133:639–48.8. Lai TY, Chan WM, Liu DT, et al. Intravitreal bevacizumab (Avastin) with or without photodynamic therapy for the treatment of polypoidal choroidal vasculopathy. Br J Ophthalmol. 2008; 92:661–6.
Article9. Augustin AJ, Puls S, Offermann I. Triple therapy for choroidal neovascularization due to age-related macular degeneration: verteporfin PDT, bevacizumab, and dexamethasone. Retina. 2007; 27:133–40.10. Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006; 355:1432–44.
Article11. Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006; 355:1419–31.
Article12. Shah GK, Sang DN, Hughes MS. Verteporfin combination regimens in the treatment of neovascular age-related macular degeneration. Retina. 2009; 29:133–48.
Article13. Michels S, Hansmann F, Geitzenauer W, Schmidt-Erfurth U. Influence of treatment parameters on selectivity of verteporfin therapy. Invest Ophthalmol Vis Sci. 2006; 47:371–6.
Article14. Schmidt-Erfurth U, Miller JW, Sickenberg M, et al. Photodynamic therapy with verteporfin for choroidal neovascularization caused by age-related macular degeneration: results of retreatments in a phase 1 and 2 study. Arch Ophthalmol. 1999; 117:1177–87.15. Smith BT, Dhalla MS, Shah GK, et al. Intravitreal injection of bevacizumab combined with verteporfin photodynamic therapy for choroidal neovascularization in age-related macular degeneration. Retina. 2008; 28:675–81.
Article16. Dhalla MS, Shah GK, Blinder KJ, et al. Combined photodynamic therapy with verteporfin and intravitreal bevacizumab for choroidal neovascularization in age-related macular degeneration. Retina. 2006; 26:988–93.
Article17. Fung AE, Lalwani GA, Rosenfeld PJ, et al. An optical coherence tomography-guided, variable dosing regimen with intravitreal ranibizumab (Lucentis) for neovascular age-related macular degeneration. Am J Ophthalmol. 2007; 143:566–83.
Article18. Shima C, Gomi F, Sawa M, et al. One-year results of combined photodynamic therapy and intravitreal bevacizumab injection for retinal pigment epithelial detachment secondary to age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2009; 247:899–906.
Article19. Ahmadieh H, Taei R, Soheilian M, et al. Single-session photo-dynamic therapy combined with intravitreal bevacizumab for neovascular age-related macular degeneration. Eur J Ophthalmol. 2008; 18:297–300.
Article20. Ruamviboonsuk P, Tadarati M, Vanichvaranont S, et al. Photodynamic therapy combined with ranibizumab for polypoidal choroidal vasculopathy: results of a 1-year preliminary study. Br J Ophthalmol. 2010; 94:1045–51.
Article21. Okubo A, Sameshima M, Uemura A, et al. Clinicopathological correlation of polypoidal choroidal vasculopathy revealed by ultrastructural study. Br J Ophthalmol. 2002; 86:1093–8.
Article22. Costa RA, Navajas EV, Farah ME, et al. Polypoidal choroidal vasculopathy: angiographic characterization of the network vascular elements and a new treatment paradigm. Prog Retin Eye Res. 2005; 24:560–86.
Article23. Lee PY, Kim KS, Lee WK. Photodynamic therapy with verteporfin in polypoidal choroidal vasculopathy. J Korean Ophthalmol Soc. 2004; 45:216–27.24. Lee SY, Kim JG, Joe SG, et al. The therapeutic effects of bevacizumab in patients with polypoidal choroidal vasculopathy. Korean J Ophthalmol. 2008; 22:92–9.
Article25. Kaiser PK. Verteporfin photodynamic therapy combined with intravitreal bevacizumab for neovascular age-related macular degeneration. Ophthalmology. 2009; 116:747–55.
Article26. Hara R, Kawaji T, Inomata Y, et al. Photodynamic therapy alone versus combined with intravitreal bevacizumab for neovascular age-related macular degeneration without polypoidal choroidal vasculopathy in Japanese patients. Graefes Arch Clin Exp Ophthalmol. 2010; 248:931–6.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Treatment of Exudative Age-Related Macular Degeneration
- Photodynamic Therapy with Verteporfin in Polypoidal Choroidal Vasculopathy
- Peripapillary Choroidal Thickness Change of Polypoidal Choroidal Vasculopathy after Anti-vascular Endothelial Growth Factor
- Multifocal Electroretinogram Findings after Intravitreal Bevacizumab Injection in Choroidal Neovascularization of Age-Related Macular Degeneration
- Intravitreal Bevacizumab With or Without Photodynamic Therapy for the Treatment of Polypoidal Choroidal Vasculopathy