Korean J Ophthalmol.
2011 Jun;25(3):161-165. 10.3341/kjo.2011.25.3.161.
Multifocal Electroretinogram Findings after Intravitreal Bevacizumab Injection in Choroidal Neovascularization of Age-Related Macular Degeneration
- Affiliations
-
- 1Department of Ophthalmology, Soonchunhyang University College of Medicine, Bucheon, Korea. yhohn@schmc.ac.kr
- KMID: 1010022
- DOI: http://doi.org/10.3341/kjo.2011.25.3.161
Abstract
- PURPOSE
To evaluate the changes in multifocal electroretinogram (mfERG) and optical coherence tomography (OCT) after intravitreal bevacizumab injection in the treatment of age-related macular degeneration (AMD).
METHODS
Twenty-one eyes with choroidal neovascularization secondary to AMD were studied before and after intravitreal bevacizumab injection for best corrected visual acuity (BCVA), OCT, and mfERG.
RESULTS
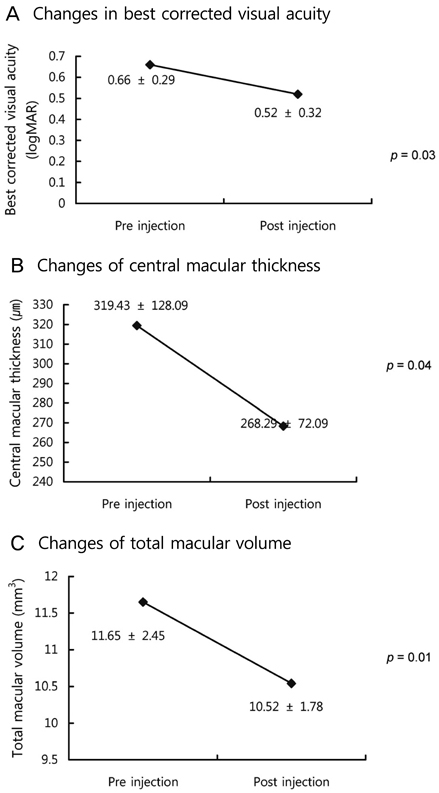
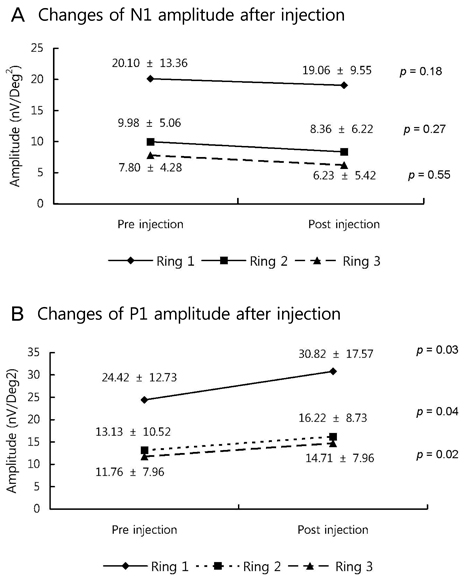
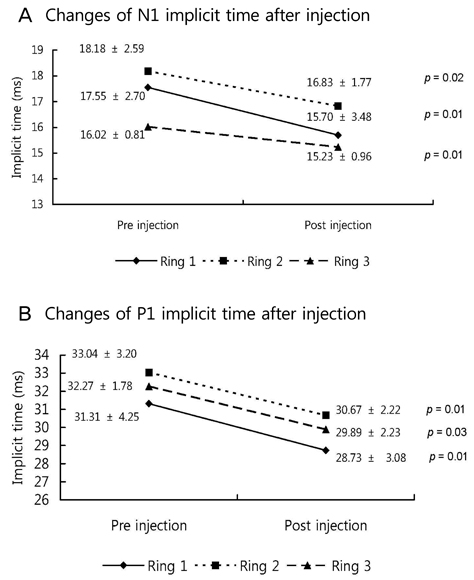
The BCVA improved, while central macular thickness and total macular volume in OCT decreased after intravitreal bevacizumab injection (p = 0.03, 0.01, and 0.01, respectively). In mfERG, the amplitude of P1, and implicit time of P1 and N1 indicated a statistically significant improvement of retinal response after intravitreal bevacizumab injection.
CONCLUSIONS
There is a potential role for mfERG in evaluating the effect on retinal function of intravitreal bevacizumab injection.
Keyword
MeSH Terms
-
Adult
Aged
Angiogenesis Inhibitors/*administration & dosage
Antibodies, Monoclonal/*administration & dosage
Choroidal Neovascularization/*drug therapy/*etiology
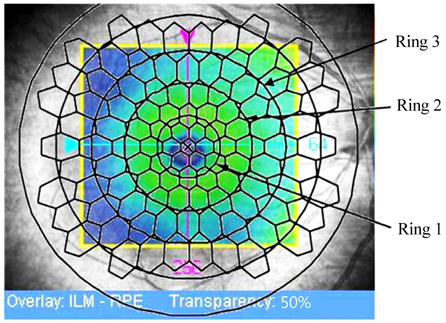
Electroretinography/*methods
Eyeglasses
Humans
Intravitreal Injections
Macular Degeneration/*complications/diagnosis/physiopathology
Middle Aged
Retina/drug effects/physiopathology
Tomography, Optical Coherence
Visual Acuity
Figure
Reference
-
1. Friedman DS, O'Colmain BJ, Muñoz B, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004. 122:564–572.2. Kroll P, Meyer CH. Which treatment is best for which AMD patient? Br J Ophthalmol. 2006. 90:128–130.3. Adamis AP, Shima DT. The role of vascular endothelial growth factor in ocular health and disease. Retina. 2005. 25:111–118.4. Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004. 25:581–611.5. Cohen SY. Anti-VEGF drugs as the 2009 first-line therapy for choroidal neovascularization in pathologic myopia. Retina. 2009. 29:1062–1066.6. Modarres M, Naseripour M, Falavarjani KG, et al. Intravitreal injection of 2.5 mg versus 1.25 mg bevacizumab (Avastin) for treatment of CNV associated with AMD. Retina. 2009. 29:319–324.7. Tatar O, Yoeruek E, Szurman P, et al. Effect of bevacizumab on inflammation and proliferation in human choroidal neovascularization. Arch Ophthalmol. 2008. 126:782–790.8. Schouten JS, La Heij EC, Webers CA, et al. A systematic review on the effect of bevacizumab in exudative age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2009. 247:1–11.9. Avery RL, Pieramici DJ, Rabena MD, et al. Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmology. 2006. 113:363–372.e5.10. Rich RM, Rosenfeld PJ, Puliafito CA, et al. Short-term safety and efficacy of intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Retina. 2006. 26:495–511.11. Kim YH, Kim ES, Yu SY, Kwak HW. Long-term effect of intravitreal bevacizumab for CNV secondary to age-related macular degeneration. J Korean Ophthalmol Soc. 2008. 49:1935–1940.12. Karanjia R, Eng KT, Gale J, et al. Electrophysiological effects of intravitreal Avastin (bevacizumab) in the treatment of exudative age-related macular degeneration. Br J Ophthalmol. 2008. 92:1248–1252.13. Moschos MM, Brouzas D, Apostolopoulos M, et al. Intravitreal use of bevacizumab (Avastin) for choroidal neovascularization due to ARMD: a preliminary multifocal-ERG and OCT study. Multifocal-ERG after use of bevacizumab in ARMD. Doc Ophthalmol. 2007. 114:37–44.14. Maturi RK, Bleau LA, Wilson DL. Electrophysiologic findings after intravitreal bevacizumab (Avastin) treatment. Retina. 2006. 26:270–274.15. Berrow EJ, Bartlett HE, Eperjesi F, Gibson JM. The electroretinogram: a useful tool for evaluating age-related macular disease? Doc Ophthalmol. 2010. 121:51–62.16. Oh SB, Cho WB, Moon JW, Kim HC. Effects and prognostic factors of intravitreal bevacizumab injection on choroidal neovascularization from age-related macular degeneration. J Korean Ophthalmol Soc. 2009. 50:202–210.17. Maturi RK, Yu M. Ciulla TA, Regillo CD, Harris A, editors. Multifocal electroretinography and its clinical application. Retina and optic nerve imaging. 2003. Philadelphia: Lippincott Williams & Wilkins;213–230.18. Hood DC, Frishman LJ, Saszik S, Viswanathan S. Retinal origins of the primate multifocal ERG: implications for the human response. Invest Ophthalmol Vis Sci. 2002. 43:1673–1685.19. Hood DC. Assessing retinal function with the multifocal technique. Prog Retin Eye Res. 2000. 19:607–646.20. Marmor MF, Tan F, Sutter EE, Bearse MA Jr. Topography of cone electrophysiology in the enhanced S cone syndrome. Invest Ophthalmol Vis Sci. 1999. 40:1866–1873.21. Kretschmann U, Stilling R, Rüther K, Zrenner E. Familial macular cone dystrophy: diagnostic value of multifocal ERG and two-color threshold perimetry. Graefes Arch Clin Exp Ophthalmol. 1999. 237:429–432.22. Li J, Tso MO, Lam TT. Reduced amplitude and delayed latency in foveal response of multifocal electroretinogram in early age related macular degeneration. Br J Ophthalmol. 2001. 85:287–290.23. Kondo M, Miyake Y, Horiguchi M, et al. Clinical evaluation of multifocal electroretinogram. Invest Ophthalmol Vis Sci. 1995. 36:2146–2150.24. Schneck ME, Bearse MA Jr, Han Y, et al. Comparison of mfERG waveform components and implicit time measurement techniques for detecting functional change in early diabetic eye disease. Doc Ophthalmol. 2004. 108:223–230.25. Hood DC, Holopigian K, Greenstein V, et al. Assessment of local retinal function in patients with retinitis pigmentosa using the multi-focal ERG technique. Vision Res. 1998. 38:163–179.26. Seeliger M, Kretschmann U, Apfelstedt-Sylla E, et al. Multifocal electroretinography in retinitis pigmentosa. Am J Ophthalmol. 1998. 125:214–226.27. Miyake Y, Horiguchi M, Tomita N, et al. Occult macular dystrophy. Am J Ophthalmol. 1996. 122:644–653.28. Hood DC, Odel JG, Chen CS, Winn BJ. The multifocal electroretinogram. J Neuroophthalmol. 2003. 23:225–235.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Electrophysiological and Morphological Changes After Intravitreal Bevacizumab Injection with Macular Edema or Choroidal Neovascularization
- Effect of High-dose Intravitreal Bevacizumab Injection on Refractory Idiopathic Choroidal Neovasculariz
- Effects and Prognostic Factors of Intravitreal Bevacizumab Injection on Choroidal Neovascularization from Age-Related Macular Degeneration
- Long-term Effect of Intravitreal Bevacizumab for CNV Secondary to Age-Related Macular Degeneration
- Full-thickness Macular Hole after Intravitreal Aflibercept Injection in a Patient with Wet Age-related Macular Degeneration