J Korean Ophthalmol Soc.
2009 Jul;50(7):1105-1110. 10.3341/jkos.2009.50.7.1105.
Upper Eyelid Reconstruction Using the Medpor(R) Sheet and Median Forehead Flap
- Affiliations
-
- 1Department of Ophthalmology, College of Medicine, Keimyung University, Daegu, Korea. changsd@dsmc.or.kr
- KMID: 2212502
- DOI: http://doi.org/10.3341/jkos.2009.50.7.1105
Abstract
- PURPOSE
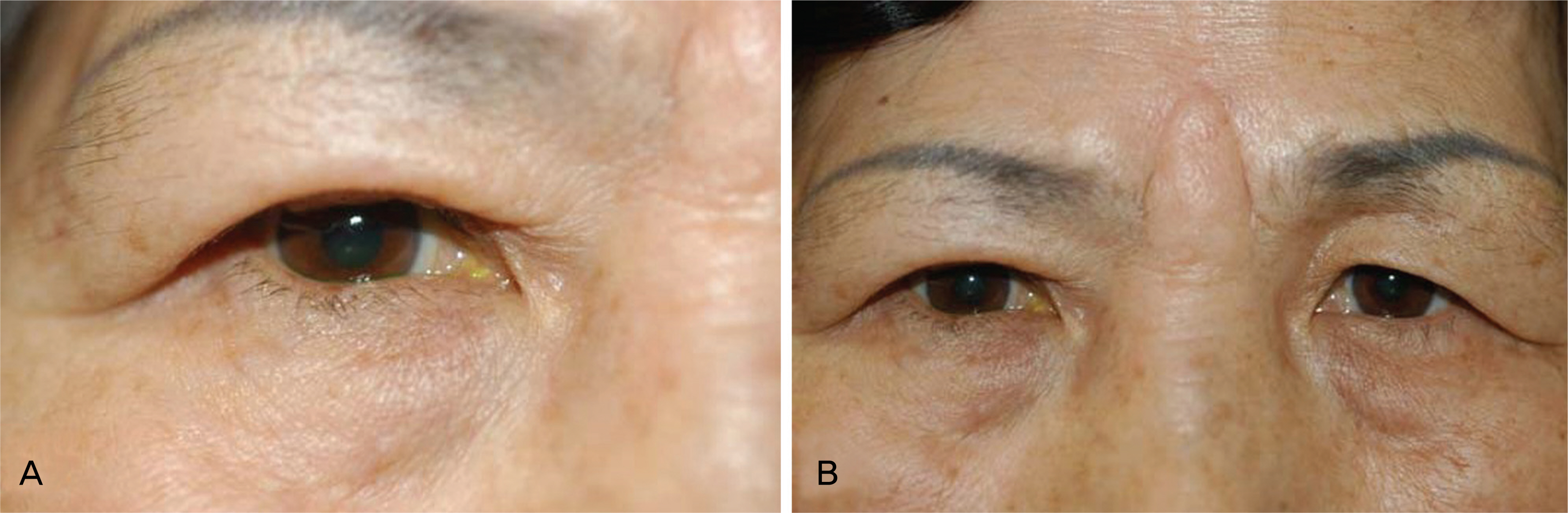
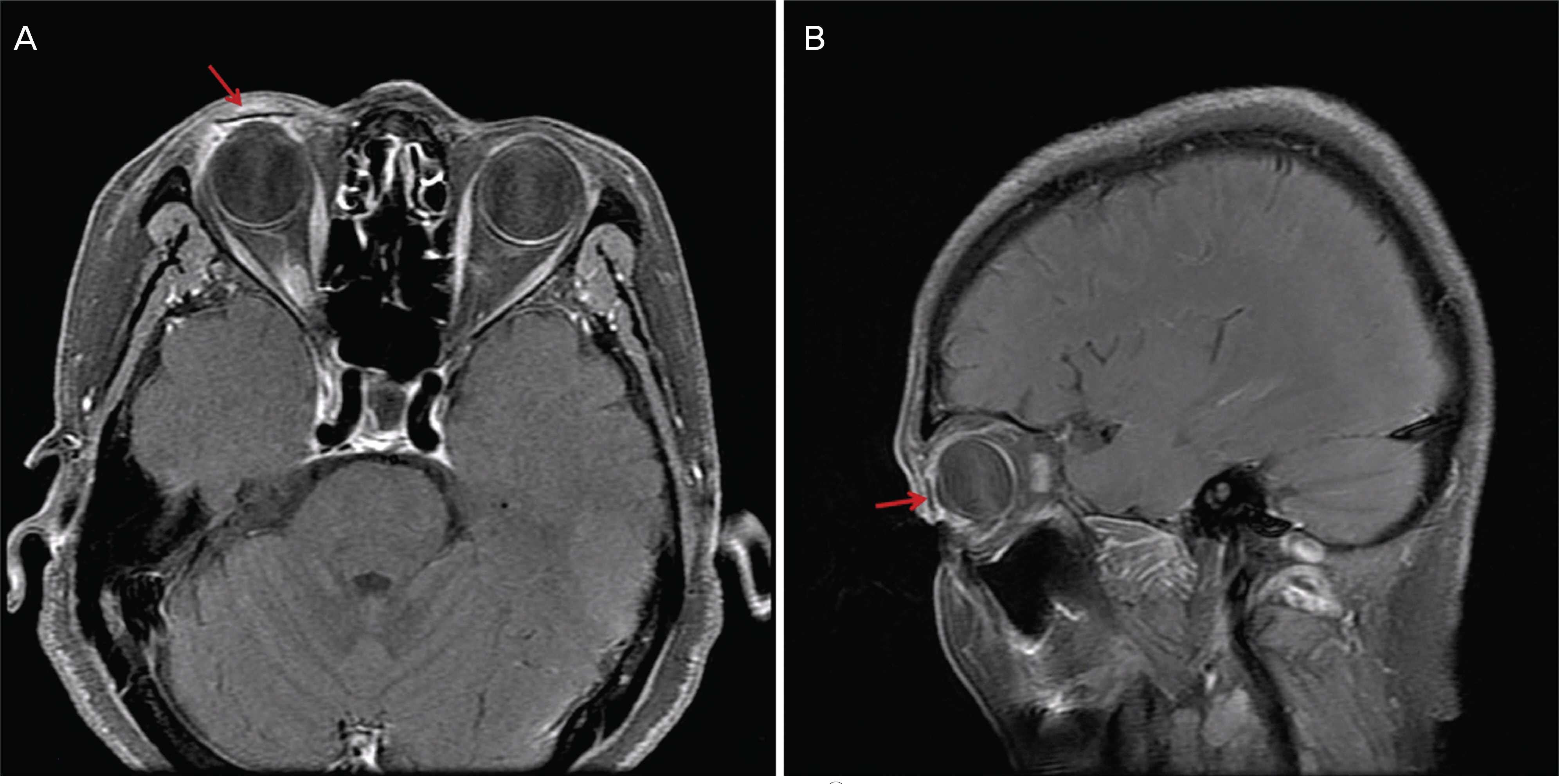
To report the upper eyelid reconstruction with median forehead flap and Medpor(R) sheet implant following full-thickness eyelid defect resulting from tumor resection. CASE SUMMARY: A 65-year-old woman was examined at our hospital for a recurrent mass on the right upper eyelid. A wide tumor excision with a 3 mm margin and an eyelid reconstruction procedure were performed after a frozen section biopsy revealed a malignancy. The full-thickness eyelid defect was reconstructed with a median forehead flap as a substitute for skin and muscle and a Medpor(R) sheet substituted for tarsal plate was sutured with a lower conjunctiva flap for posterior lamella. The histopathology diagnosis of the lesion showed a well-differentiated squamous cell carcinoma. The lower conjunctiva was separated at 2 weeks after surgery, and then the median forehead flap detached from the upper eyelid skin at 8 weeks after surgery. One year postoperatively, there was no evidence of a wound infection and an exposure of the Medpor(R) sheet. The eyelid had a good cosmetic contour and the movement of the eyelid during blinking was natural. CONCLUSIONS: The use of a Medpor(R) sheet as a substitute for a tarsal plate in reconstructive procedures of the upper eyelid defect shows cosmetic and functional success.
MeSH Terms
Figure
Cited by 1 articles
-
The Effects of Commodified Growth Factor Products on the Fibrovascularization of Porous Polyethylene Orbital Implants
Won Mo Gu, Joon Hyuk Choi, Jun Hyuk Son
J Korean Ophthalmol Soc. 2014;55(9):1366-1371. doi: 10.3341/jkos.2014.55.9.1366.
Reference
-
References
1. McCord CD Jr, Wesley R. Reconstruction of the upper eyelid and medial canthus. McCord CD, Tanenbaum M., editorsOculoplastic Surgery. 2nd ed.New York: Raven Press;1987. chap.p. 3.2. Remano JJ, Iliff NT, Manson PN. Use of Medpor porous polyethylene implants in 140 patients with facial fracture. J Craniofac Surg. 1993; 4:142–7.3. Karesh JW, Dresner SC. High-density porous polyethylene (Medpor) as a successful anophthalmic socket implant. Ophthalmology. 1994; 101:1688–96.
Article4. Alwitry A, West S, King J, et al. Long term follow-up porous polyethylene spherical implants after enucleation and evisceration. Ophthal Plast reconstr Surg. 2007; 23:11–5.5. Odum BC, Bussard GM, Lewis RP, et al. High-density porous polyethylene for facial bone augmentation. J Long Term Eff Med Implant. 1998; 891:3–17.6. McCord CD Jr, Codner MA. Free tissue graft. McCord CD, editorEyelid surgery: principles and techniques. 3rd ed.Philadelphia: Lippincott-Raven;1995. chap.p. 3.7. Lee HB, Kwon KY, Chung SK. Reconstruction of the lower eyelid utilizing a chondromucosa after removal of squamous cell carcinoma. J Korean Ophthalmol Soc. 1985; 26:111–5.8. Park WC, Han SK, Kim NJ, et al. Effect of basic fibroblast growth factor on fibrovascular ingrowth into porous polyethylene anophthalmic socket implants. Korean J Ophthalmol. 2005; 19:1–8.
Article9. Kim HS, Ryu YA, Woo JS, et al. Comparative observation of barrier sheet and nonbarrier sheet Medpor® inserted on orbital floor in rabbits. J Korean Soc Plast Reconstr Surg. 2004; 31:682–6.10. Lim J-Y, Jang JW, Moon SH. Lid reconstruction using the porous polyethylene (Medpor®) sheet. J Korean Ophthalmol Soc. 2003; 44:2111–6.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Medpor(R) Sheet as Substitute for Tarsus in Eyelid Reconstruction
- Lid Reconstruction Using the Porous Polyethylene (Medpor(R)) Sheet
- Simultaneous Upper and Lower Eyelid Reconstruction for Eyelid Defects Following a Dog Bite
- Clinical Experience of Orbital wall Reconstruction using Medpor(R) Barrier Sheet Implant
- Comparative Observation of Barrier Sheet and Nonbarrier Sheet Medpor(R) Inserted on Orbital Floor in Rabbits