J Korean Surg Soc.
2010 May;78(5):283-289. 10.4174/jkss.2010.78.5.283.
Association of RNase3 Polymorphisms with the Susceptibility of Gastric Cancer
- Affiliations
-
- 1Department of Surgery, Institute of Medical Science, College of Medicine, Wonkwang University, Iksan, Korea. rjk@wonkwang.ac.kr
- 2Department of Radiology, Institute of Medical Science, College of Medicine, Wonkwang University, Iksan, Korea.
- 3Department of Pathology, Institute of Medical Science, College of Medicine, Wonkwang University, Iksan, Korea.
- KMID: 2211954
- DOI: http://doi.org/10.4174/jkss.2010.78.5.283
Abstract
- PURPOSE
RNase3 is a secretory ribonuclease, which is found in the eosinophilic leukocyte and involved in the innate immune system. Its cytotoxic activity is effective against a wide range of pathogens. We performed a case-control study to examine the relationship between RNase3 polymorphisms and the susceptibility of gastric cancer in Korean people.
METHODS
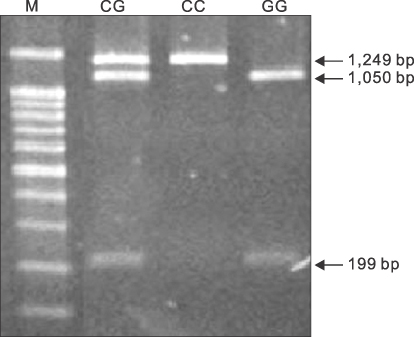
Blood sampling of stomach cancer and healthy persons groups were performed, Taqman in g.-550A>G, polymerase chain reaction-restriction fragment length polymorphism in g.371C>G, and high-resolution melt in g.499C>G were analyzed. The three single nucleotide polymorphisms g.-550A>G, g.371C>G, and g.499C>G in RNase3 and their haplotypes were analyzed.
RESULTS
The genotype and allele frequencies of RNase3 g.-550A>G and g.371C>G were not significantly increased in susceptibility of gastric cancer than control group. But, RNase3 CC genotype was associated with a significantly increased susceptibility of gastric cancer than control group (P=0.002). Also, RNase3 CC genotype was more specifically associated with a significantly increased susceptibility of middle and lower gastric cancer than upper gastric cancer (P=0.002). In haplotype of RNase3 SNP g.-550A, g.371G, and g.499C, there was significantly susceptibility of gastric cancer (P=0.004), and more specific influence on middle and lower gastric cancer than upper gastric cancer (P=0.006 vs 0.054).
CONCLUSION
RNase3 g.499C>G polymorphism may influence gastric cancers, and have a more specific influence on middle and lower gastric cancer rather than upper gastric cancer. But RNase3 g.-550A>G, g.371C>G polymorphisms need careful interpretation and confirmation in more larger studies.
Keyword
MeSH Terms
Figure
Reference
-
1. Hood L, Galas D. The digital code of DNA. Nature. 2003. 421:444–448.2. Salisbury BA, Pungliya M, Choi JY, Jiang R, Sun XJ, Stephens JC. SNP and haplotype variation in the human genome. Mutat Res. 2003. 526:53–61.3. Gianfagna F, De Feo E, van Duijn CM, Ricciardi G, Boccia S. A systematic review of meta-analyses on gene polymorphisms and gastric cancer risk. Curr Genomics. 2008. 9:361–374.4. Hartgrink HH, Jansen EP, van Grieken NC, van de Velde CJ. Gastric cancer. Lancet. 2009. 374:477–490.5. The Information Committee of the Korean Gastric Cancer Association. 2004 Nationwide gastric cancer report in Korea. J Korean Gastric Cancer Assoc. 2007. 7:47–54.6. Boix E, Torrent M, Sanchez D, Nogues MV. The antipathogen activities of eosinophil cationic protein. Curr Pharm Biotechnol. 2008. 9:141–152.7. Navarro S, Aleu J, Jimenez M, Boix E, Cuchillo CM, Nogues MV. The cytotoxicity of eosinophil cationic protein/ribonuclease 3 on eukaryotic cell lines takes place through its aggregation on the cell membrane. Cell Mol Life Sci. 2008. 65:324–337.8. Amin K, Ludviksdottir D, Janson C, Nettelbladt O, Bjornsson E, Roomans GM, et al. Inflammation and structural changes in the airways of patients with atopic and nonatopic asthma. BHR Group. Am J Respir Crit Care Med. 2000. 162:2295–2301.9. Jonsson UB, Bystrom J, Stalenheim G, Venge P. Polymorphism of the eosinophil cationic protein-gene is related to the expression of allergic symptoms. Clin Exp Allergy. 2002. 32:1092–1095.10. Munthe-Kaas MC, Gerritsen J, Carlsen KH, Undlien D, Egeland T, Skinningsrud B, et al. Eosinophil cationic protein (ECP) polymorphisms and association with asthma, s-ECP levels and related phenotypes. Allergy. 2007. 62:429–436.11. Rubin J, Zagai U, Blom K, Trulson A, Engstrom A, Venge P. The coding ECP 434(G>C) gene polymorphism determines the cytotoxicity of ECP but has minor effects on fibroblast-mediated gel contraction and no effect on RNase activity. J Immunol. 2009. 183:445–451.12. Maeda T, Mahara K, Kitazoe M, Futami J, Takidani A, Kosaka M, et al. RNase 3 (ECP) is an extraordinarily stable protein among human pancreatic-type RNases. J Biochem. 2002. 132:737–742.13. Tajima K, Yamakawa M, Inaba Y, Katagiri T, Sasaki H. Cellular localization of interleukin-5 expression in rectal carcinoma with eosinophilia. Hum Pathol. 1998. 29:1024–1028.14. Balasubramanian SP, Cox A, Brown NJ, Reed MW. Candidate gene polymorphisms in solid cancers. Eur J Surg Oncol. 2004. 30:593–601.15. Stephens JC. Single-nucleotide polymorphisms, haplotypes, and their relevance to pharmacogenetics. Mol Diagn. 1999. 4:309–317.16. Kreuze JF, Savenkov EI, Cuellar W, Li X, Valkonen JP. Viral class 1 RNase III involved in suppression of RNA silencing. J Virol. 2005. 79:7227–7238.17. Taha Y, Raab Y, Carlson M, Larsson A, Lordal M, Loof L, et al. Steroids reduce local inflammatory mediator secretion and mucosal permeability in collagenous colitis patients. World J Gastroenterol. 2006. 12:7012–7018.18. Shichijo K, Makiyama K, Wen CY, Matsuu M, Nakayama T, Nakashima M, et al. Antibody to eosinophil cationic protein suppresses dextran sulfate sodium-induced colitis in rats. World J Gastroenterol. 2005. 11:4505–4510.19. von Wasielewski R, Seth S, Franklin J, Fischer R, Hubner K, Hansmann ML, et al. Tissue eosinophilia correlates strongly with poor prognosis in nodular sclerosing Hodgkin's disease, allowing for known prognostic factors. Blood. 2000. 95:1207–1213.20. Molin D, Glimelius B, Sundstrom C, Venge P, Enblad G. The serum levels of eosinophil cationic protein (ECP) are related to the infiltration of eosinophils in the tumours of patients with Hodgkin's disease. Leuk Lymphoma. 2001. 42:457–465.21. Trulson A, Nilsson S, Venge P. The eosinophil granule proteins in serum, but not the oxidative metabolism of the blood eosinophils, are increased in cancer. Br J Haematol. 1997. 98:312–314.22. Trulson A, Bystrom J, Engstrom A, Larsson R, Venge P. The functional heterogeneity of eosinophil cationic protein is determined by a gene polymorphism and post-translational modifications. Clin Exp Allergy. 2007. 37:208–218.23. Enblad G, Sundstrom C, Glimelius B. Infiltration of eosinophils in Hodgkin's disease involved lymph nodes predicts prognosis. Hematol Oncol. 1993. 11:187–193.24. Stephens JC, Schneider JA, Tanguay DA, Choi J, Acharya T, Stanley SE, et al. Haplotype variation and linkage disequilibrium in 313 human genes. Science. 2001. 293:489–493.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Association of RNase3 Polymorphisms with the Risk of Colorectal Cancer
- Analysis of CYP1A1 , GST-mu and GST-theta Polymorphisms for the Determination of Genetic Susceptibility to Korean Gastric Cancer
- Gastric Cancer Susceptibility according to Methylenetetrahydrofolate Reductase and Thymidylate Synthase Gene Polymorphism
- Association between genetic polymorphisms in cortactin and susceptibility to gastric cancer
- The Effects of CYP2E1 and CYP2C19 Polymorphisms on the Susceptibility for Gastric Cancer