J Korean Surg Soc.
2010 Mar;78(3):177-183. 10.4174/jkss.2010.78.3.177.
Cyclooxygenase (COX)-1, Cyclooxygenase (COX)-2 and E-cadherin Expression in Colorectal Cancer Patients with Hepatic Metastasis
- Affiliations
-
- 1Department of Surgery, School of Medicine, Kyung Hee University, Seoul, Korea. sunhyung@chol.com
- 2Department of Pathology, School of Medicine, Kyung Hee University, Seoul, Korea.
- KMID: 2211946
- DOI: http://doi.org/10.4174/jkss.2010.78.3.177
Abstract
- PURPOSE
Recent studies have shown that cyclooxygenase (COX)-2 may be involved in colorectal carcinogenesis. In this study, we evaluate the differences of COX-2 expression in patients with synchronous and metachronous hepatic metastasis of colorectal cancer. In addition, the expression of COX-1 and E-cadherin were also evaluated.
METHODS
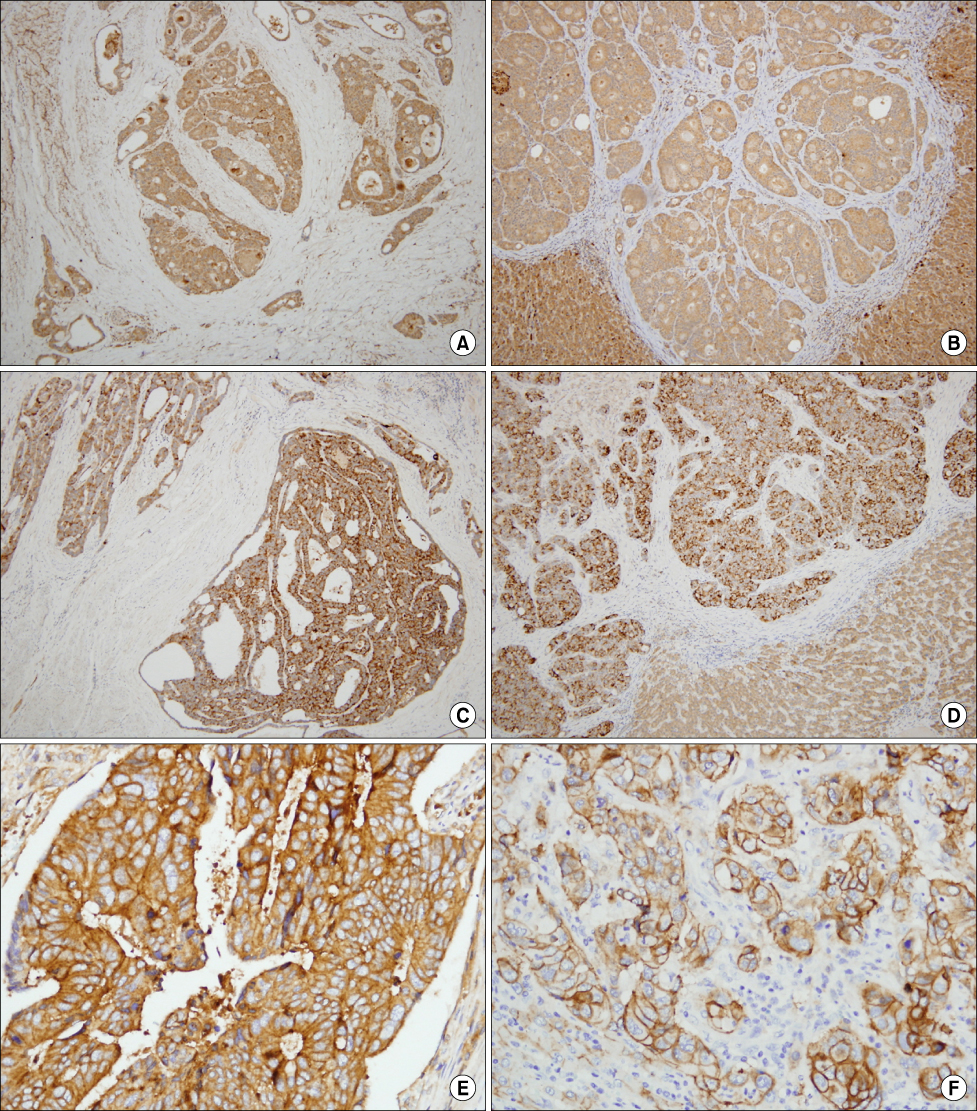
Paraffin embedded blocks were obtained from 41 patients who underwent surgery for colorectal cancer with hepatic metastasis. Samples from primary colorectal cancer, synchronous and metachronous hepatic lesions were stained by immunohistochemistry for monoclonal antibody against COX-1, COX-2, and E-cadherin.
RESULTS
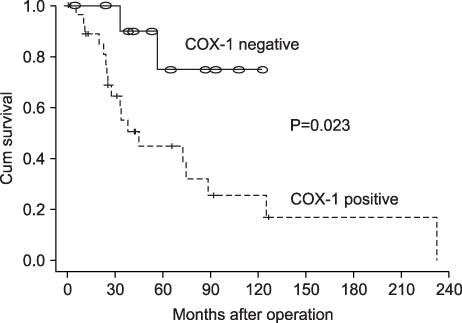
In colonic COX-1 expression, there was no significant difference in the degree of COX-1 expression between primary colorectal cancer with synchronous hepatic metastasis and that of metachronous hepatic metastasis (P=0.507). In colonic COX-2 and E-cadherin expression, the degree of COX-2 expression was not different between the two groups. But, the patient survival rate in the positive group of COX-1 and COX-2 expression was lower than in the negative group, respectively (P=0.023, P=0.006).
CONCLUSION
The degree of colonic COX-1 and COX-2 expression has an impact on prognosis in synchronous and metachronous hepatic metastasis. Further large-scale study is necessary to determine the meaning of COX-2 expression in colorectal cancer.
Keyword
MeSH Terms
Figure
Reference
-
1. Bengmark S, Hafstrom L. The natural history of primary and secondary malignant tumors of the liver. I. The prognosis for patients with hepatic metastases from colonic and rectal carcinoma by laparotomy. Cancer. 1969. 23:198–202.2. Abdalla EK, Vauthey JN, Ellis LM, Ellis V, Pollock R, Broglio KR, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg. 2004. 239:818–827.3. Ohlsson B, Stenram U, Tranberg KG. Resection of colorectal liver metastases: 25-year experience. World J Surg. 1998. 22:268–277.4. Ekberg H, Tranberg KG, Andersson R, Lundstedt C, Hagerstrand I, Ranstam J, et al. Pattern of recurrence in liver resection for colorectal secondaries. World J Surg. 1987. 11:541–547.5. Bozzetti F, Doci R, Bignami P, Morabito A, Gennari L. Patterns of failure following surgical resection of colorectal cancer liver metastases. Rationale for a multimodal approach. Ann Surg. 1987. 205:264–270.6. Scheele J, Stangl R, Altendorf-Hofmann A, Gall FP. Indicators of prognosis after hepatic resection for colorectal secondaries. Surgery. 1991. 110:13–29.7. Nordlinger B, Guiguet M, Vaillant JC, Balladur P, Boudjema K, Bachellier P, et al. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Francaise de Chirurgie. Cancer. 1996. 77:1254–1262.8. Rao CV, Rivenson A, Simi B, Zang E, Kelloff G, Steele V, et al. Chemoprevention of colon carcinogenesis by sulindac, a nonsteroidal anti-inflammatory agent. Cancer Res. 1995. 55:1464–1472.9. Thun MJ, Namboodiri MM, Heath CW Jr. Aspirin use and reduced risk of fatal colon cancer. N Engl J Med. 1991. 325:1593–1596.10. Smalley WE, DuBois RN. Colorectal cancer and nonsteroidal anti-inflammatory drugs. Adv Pharmacol. 1997. 39:1–20.11. Yamauchi T, Watanabe M, Kubota T, Hasegawa H, Ishii Y, Endo T, et al. Cyclooxygenase-2 expression as a new marker for patients with colorectal cancer. Dis Colon Rectum. 2002. 45:98–103.12. Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971. 231:232–235.13. Kujubu DA, Fletcher BS, Varnum BC, Lim RW, Herschman HR. TIS10, a phorbol ester tumor promoter-inducible mRNA from Swiss 3T3 cells, encodes a novel prostaglandin synthase/cyclooxygenase homologue. J Biol Chem. 1991. 266:12866–12872.14. Hla T, Neilson K. Human cyclooxygenase-2 cDNA. Proc Natl Acad Sci U S A. 1992. 89:7384–7388.15. DuBois RN, Tsujii M, Bishop P, Awad JA, Makita K, Lanahan A. Cloning and characterization of a growth factor-inducible cyclooxygenase gene from rat intestinal epithelial cells. Am J Physiol. 1994. 266:G822–G827.16. Ristimaki A, Honkanen N, Jankala H, Sipponen P, Harkonen M. Expression of cyclooxygenase-2 in human gastric carcinoma. Cancer Res. 1997. 57:1276–1280.17. Eberhart CE, Coffey RJ, Radhika A, Giardiello FM, Ferrenbach S, DuBois RN. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology. 1994. 107:1183–1188.18. Sano H, Kawahito Y, Wilder RL, Hashiramoto A, Mukai S, Asai K, et al. Expression of cyclooxygenase-1 and -2 in human colorectal cancer. Cancer Res. 1995. 55:3785–3789.19. Tsujii M, DuBois RN. Alterations in cellular adhesion and apoptosis in epithelial cells overexpressing prostaglandin endoperoxide synthase 2. Cell. 1995. 83:493–501.20. Tsujii M, Kawano S, Tsuji S, Sawaoka H, Hori M, DuBois RN. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell. 1998. 93:705–716.21. Soumaoro LT, Uetake H, Higuchi T, Takagi Y, Enomoto M, Sugihara K. Cyclooxygenase-2 expression: a significant prognostic indicator for patients with colorectal cancer. Clin Cancer Res. 2004. 10:8465–8471.22. Sheehan KM, Sheahan K, O'Donoghue DP, MacSweeney F, Conroy RM, Fitzgerald DJ, et al. The relationship between cyclooxygenase-2 expression and colorectal cancer. JAMA. 1999. 282:1254–1257.23. Takeichi M. The cadherins: cell-cell adhesion molecules controlling animal morphogenesis. Development. 1988. 102:639–655.24. Gofuku J, Shiozaki H, Tsujinaka T, Inoue M, Tamura S, Doki Y, et al. Expression of E-cadherin and alpha-catenin in patients with colorectal carcinoma. Correlation with cancer invasion and metastasis. Am J Clin Pathol. 1999. 111:29–37.25. Ghadimi BM, Behrens J, Hoffmann I, Haensch W, Birchmeier W, Schlag PM. Immunohistological analysis of E-cadherin, alpha-, beta- and gamma-catenin expression in colorectal cancer: implications for cell adhesion and signaling. Eur J Cancer. 1999. 35:60–65.26. Takayama T, Shiozaki H, Doki Y, Oka H, Inoue M, Yamamoto M, et al. Aberrant expression and phosphorylation of beta-catenin in human colorectal cancer. Br J Cancer. 1998. 77:605–613.27. Hull MA, Fenwick SW, Chapple KS, Scott N, Toogood GJ, Lodge JP. Cyclooxygenase-2 expression in colorectal cancer liver metastases. Clin Exp Metastasis. 2000. 18:21–27.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cyclooxygenase-2 Expression in Colorectal Cancer
- The Clinical Significance of Cyclooxygenase 2 Expression in Colorectal Cancer
- Expression of Cyclooxygenase-2 in Colorectal Cancer and Adenoma
- Colorectal Cancer and Prostaglandin
- Cytoplasmic Expression of HuR is Related to Cyclooxygenase-2 Expression in Colon Cancer