J Pathol Transl Med.
2016 Jan;50(1):58-66. 10.4132/jptm.2015.10.10.
Evaluation of the VE1 Antibody in Thyroid Cytology Using Ex Vivo Papillary Thyroid Carcinoma Specimens
- Affiliations
-
- 1Department of Pathology, Ajou University School of Medicine, Suwon, Korea. drjhk@ajou.ac.kr
- 2Department of Surgery, Ajou University School of Medicine, Suwon, Korea.
- 3Department of Medical Genetics, Ajou University School of Medicine, Suwon, Korea.
- KMID: 2211403
- DOI: http://doi.org/10.4132/jptm.2015.10.10
Abstract
- BACKGROUND
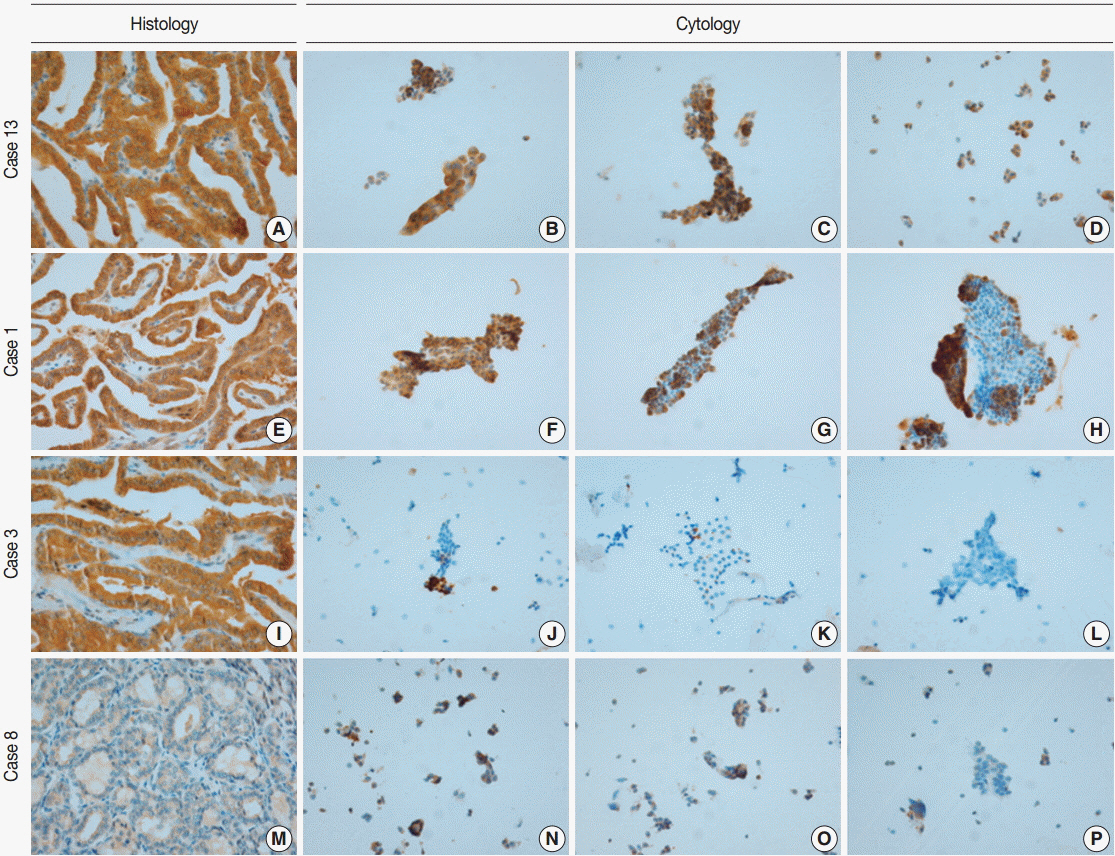
Recently, VE1, a monoclonal antibody against the BRAFV600E mutant protein, has been investigated in terms of its detection of the BRAFV600E mutation. Although VE1 immunostaining and molecular methods used to assess papillary thyroid carcinoma in surgical specimens are in good agreement, evaluation of VE1 in thyroid cytology samples is rarely performed, and its diagnostic value in cytology has not been well established. In present study, we explored VE1 immunoexpression in cytology samples from ex vivo papillary thyroid carcinoma specimens in order to minimize limitations of low cellularity and sampling/targeting errors originated from thyroid fineneedle aspiration and compared our results with those obtained using the corresponding papillary thyroid carcinoma tissues.
METHODS
The VE1 antibody was evaluated in 21 cases of thyroid cytology obtained directly from ex vivo thyroid specimens. VE1 immunostaining was performed using liquid-based cytology, and the results were compared with those obtained using the corresponding tissues.
RESULTS
Of 21 cases, 19 classic papillary thyroid carcinomas had BRAFV600E mutations, whereas two follicular variants expressed wild-type BRAF. VE1 immunoexpression varied according to specimen type. In detection of the BRAFV600E mutation, VE1 immunostaining of the surgical specimen exhibited 100% sensitivity and 100% specificity, whereas VE1 immunostaining of the cytology specimen exhibited only 94.7% sensitivity and 0% specificity.
CONCLUSIONS
Our data suggest that VE1 immunostaining of a cytology specimen is less specific than that of a surgical specimen for detection of the BRAFV600E mutation, and that VE1 immunostaining of a cytology specimen should be further evaluated and optimized for clinical use.
MeSH Terms
Figure
Reference
-
1. Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002; 417:949–54.2. Rajagopalan H, Bardelli A, Lengauer C, Kinzler KW, Vogelstein B, Velculescu VE. Tumorigenesis: RAF/RAS oncogenes and mismatch-repair status. Nature. 2002; 418:934.3. Xing M. BRAF mutation in thyroid cancer. Endocr Relat Cancer. 2005; 12:245–62.4. Kim JH, Paulus W, Heim S. BRAF V600E mutation is a useful marker for differentiating Rathke’s cleft cyst with squamous metaplasia from papillary craniopharyngioma. J Neurooncol. 2015; 123:189–91.5. Ascierto PA, Minor D, Ribas A, et al. Phase II trial (BREAK-2) of the BRAF inhibitor dabrafenib (GSK2118436) in patients with metastatic melanoma. J Clin Oncol. 2013; 31:3205–11.
Article6. Tiacci E, Trifonov V, Schiavoni G, et al. BRAF mutations in hairycell leukemia. N Engl J Med. 2011; 364:2305–15.7. Lee ST, Kim SW, Ki CS, et al. Clinical implication of highly sensitive detection of the BRAF V600E mutation in fine-needle aspirations of thyroid nodules: a comparative analysis of three molecular assays in 4585 consecutive cases in a BRAF V600E mutation-prevalent area. J Clin Endocrinol Metab. 2012; 97:2299–306.8. Kim YH, Choi SE, Yoon SO, Hong SW. A testing algorithm for detection of the B-type Raf kinase V600E mutation in papillary thyroid carcinoma. Hum Pathol. 2014; 45:1483–8.
Article9. Ritterhouse LL, Barletta JA. BRAF V600E mutation-specific antibody: a review. Semin Diagn Pathol. 2015; 32:400–8.10. Zagzag J, Pollack A, Dultz L, et al. Clinical utility of immunohistochemistry for the detection of the BRAF V600E mutation in papillary thyroid carcinoma. Surgery. 2013; 154:1199–204.11. Routhier CA, Mochel MC, Lynch K, Dias-Santagata D, Louis DN, Hoang MP. Comparison of 2 monoclonal antibodies for immunohistochemical detection of BRAF V600E mutation in malignant melanoma, pulmonary carcinoma, gastrointestinal carcinoma, thyroid carcinoma, and gliomas. Hum Pathol. 2013; 44:2563–70.12. Koperek O, Kornauth C, Capper D, et al. Immunohistochemical detection of the BRAF V600E-mutated protein in papillary thyroid carcinoma. Am J Surg Pathol. 2012; 36:844–50.13. Lee SR, Yim H, Han JH, et al. VE1 antibody is not highly specific for the BRAF V600E mutation in thyroid cytology categories with the exception of malignant cases. Am J Clin Pathol. 2015; 143:437–44.14. Wobker SE, Kim LT, Hackman TG, Dodd LG. Use of BRAF V600E immunocytochemistry on FNA direct smears of papillary thyroid carcinoma. Cancer Cytopathol. 2015; 123:531–9.15. Rossi ED, Martini M, Capodimonti S, et al. Analysis of immunocytochemical and molecular BRAF expression in thyroid carcinomas: a cytohistologic institutional experience. Cancer Cytopathol. 2014; 122:527–35.
Article16. Zimmermann AK, Camenisch U, Rechsteiner MP, Bode-Lesniewska B, Rössle M. Value of immunohistochemistry in the detection of BRAF(V600E) mutations in fine-needle aspiration biopsies of papillary thyroid carcinoma. Cancer Cytopathol. 2014; 122:48–58.17. Ghossein RA, Katabi N, Fagin JA. Immunohistochemical detection of mutated BRAF V600E supports the clonal origin of BRAF-induced thyroid cancers along the spectrum of disease progression. J Clin Endocrinol Metab. 2013; 98:E1414–21.18. Kawahara A, Taira T, Abe H, et al. Fixation effect of SurePath preservative fluids using epidermal growth factor receptor mutation-specific antibodies for immunocytochemistry. Cancer Cytopathol. 2014; 122:145–52.
Article19. Dvorak K, Aggeler B, Palting J, McKelvie P, Ruszkiewicz A, Waring P. Immunohistochemistry with the anti-BRAF V600E (VE1) antibody: impact of pre-analytical conditions and concordance with DNA sequencing in colorectal and papillary thyroid carcinoma. Pathology. 2014; 46:509–17.20. Jones RT, Abedalthagafi MS, Brahmandam M, et al. Cross-reactivity of the BRAF VE1 antibody with epitopes in axonemal dyneins leads to staining of cilia. Mod Pathol. 2015; 28:596–606.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Concurrent Medullay and Papillary Carcinoma of the Thyroid
- A Case of Mixed Follicular-Papillary Thyroid Carcinoma
- Demonstration of TCM-9 Monoclonal Antibody in Follicular Neoplasm of Thyroid
- A Case of Ectopic Thyroid Papillary Carcinoma with Incidental Papillary Thyroid Microcarcinoma
- Concurrent Papillary and Medullary Carcinoma of the Thyroid Gland