J Korean Surg Soc.
2009 Feb;76(2):73-80. 10.4174/jkss.2009.76.2.73.
Efficacy and Safety of the Electrospun Nanofibrous Adhesion Barrier for Laparoscopic Surgery in a Rabbit Model
- Affiliations
-
- 1Biorane Co., Korea.
- 2Department of Patholgogy , College of Medicine, Pochon CHA University, Seongnam, Korea.
- 3Department of General Surgery, College of Medicine, Pochon CHA University, Seongnam, Korea. wizard95@cha.ac.kr
- KMID: 2211255
- DOI: http://doi.org/10.4174/jkss.2009.76.2.73
Abstract
- PURPOSE
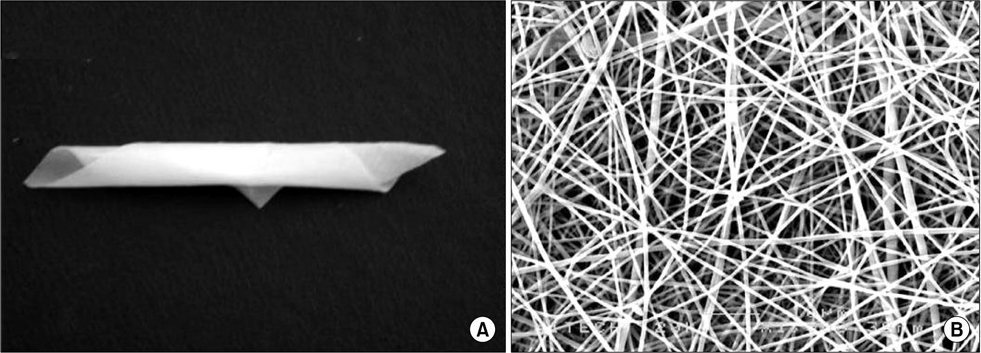
Most recently developed anti-adhesive membranes are not suitable for laparoscopic surgery due to weak mechanical properties or adhesive characteristics. To overcome these problems, we prepared electrospun bioabsorbable nanofibrous poly (lactic-co-glycolic acid)-based membranes as an adhesion barrier. We evaluated the efficacy and safety of this material for laparoscopic surgery in a rabbit model.
METHODS
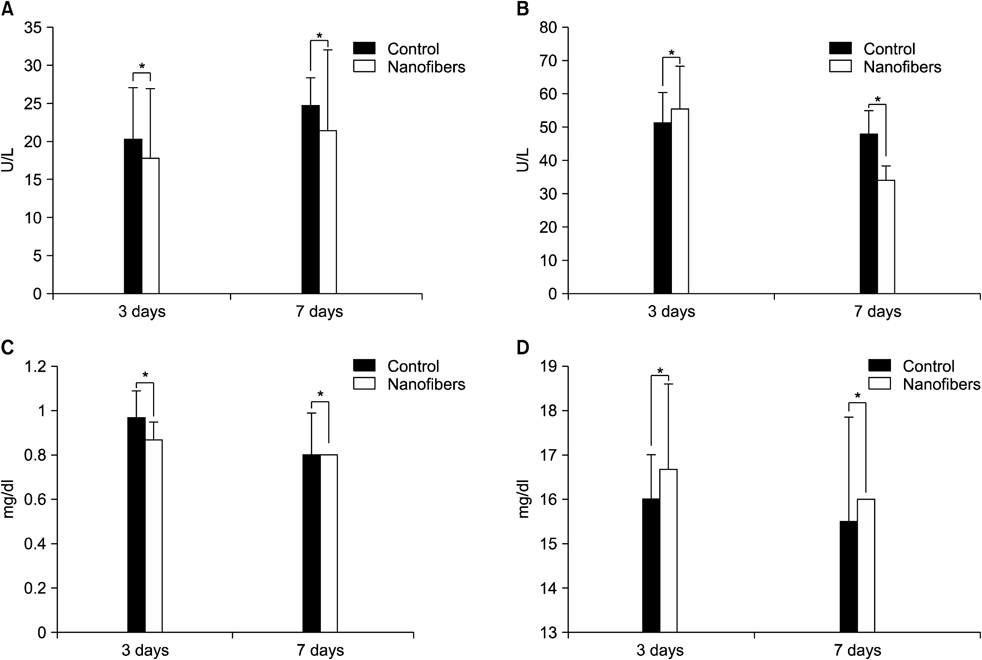
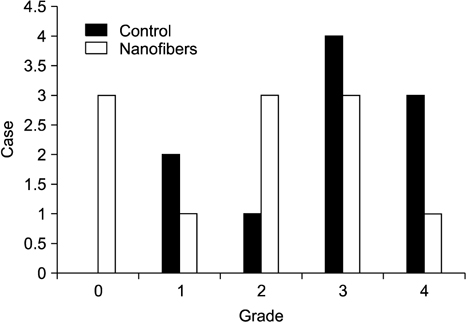
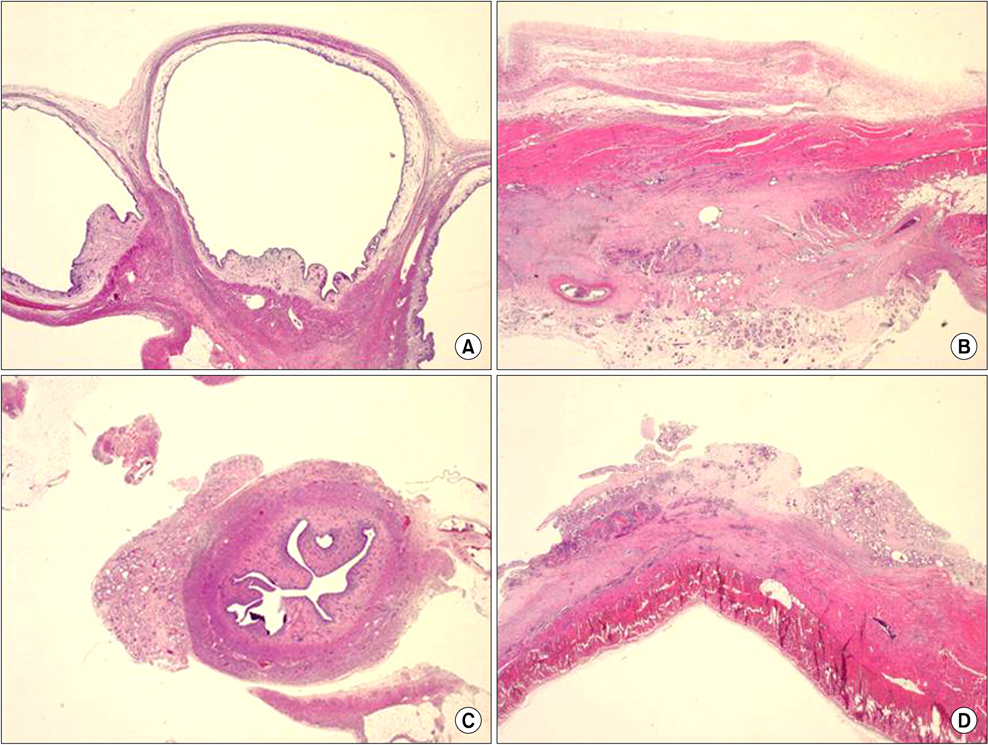
A standardized laparoscopic surgical trauma was made on the rabbit's uterine horn and adjacent abdominal wall to induce adhesion formation. The injured uterus was covered by a nanofibrous barrier or it was left untreated (the negative control group) (each group: n=14). To evaluate acute toxicity of this material, blood sampling was made 3 and 7 days after laparoscopic surgery to check liver and renal function. Three weeks after laparoscopy, a second look laparoscopy was performed and the adhesions were scored according to Blauer's scoring system. Tissue between abdominal wall and uterus was obtained to examine microscopically. Liver, kidney and uterus were harvested to examine chronic toxicity.
RESULTS
36.4% of the nanofiber treatment group and 70% of the untreated control group showed severe adhesions (grade>3) after laparoscopic surgery but failed to get a statistical significance (P=0.198). Acute and chronic toxicity induced by this material were not noted in the blood and tissue exam.
CONCLUSION
This study showed that nanofiber barrier seems to be a novel resorbable biomaterial for the reduction of postoperative adhesions. Easy placement and handling of this material make these membranes potentially successful candidates for laparoscopic surgery. But further study is needed to get a statistical significance.
Keyword
MeSH Terms
Figure
Reference
-
1. Yang HY, Suh KS, Youn YK, Kim SW, Kim SJ, Lee KU, et al. A clinical analysis of intestinal obstruction in the adult. J Korean Surg Soc. 1997. 52:335–342.2. Lee WJ, Moon BI, Park JB, Kim IM, You BO. The factors influencing the treatment for the mechanical intestinal obstruction due to adhesion. J Korean Surg Soc. 1995. 49:192–203.3. Falk K, Bjorquist P, Stromqvist M, Holmdahl L. Reduction of experimental adhesion formation by inhibition of plasminogen activator inhibitor type 1. Br J Surg. 2001. 88:286–289.4. Shim HS, Lee YW, Lee YM, Oh YH, Kwon SW, Kim JH, et al. Evaluation of resorbable materials for preventing surgical adhesion on rat experiment. J Korean Surg Soc. 2002. 63:179–186.5. Jansen RP. Failure of peritoneal irrigation with heparin during pelvic operations upon young women to reduce adhesions. Surg Gynecol Obstet. 1988. 166:154–160.6. Moll HD, Wolfe DF, Schumacher J, Wright JC. Evaluation of sodium carboxymethylcellulose for prevention of adhesions after uterine trauma in ewes. Am J Vet Res. 1992. 53:1454–1456.7. Urman B, Gomel V. Effect of hyaluronic acid on postoperative intraperitoneal adhesion formation and reformation in the rat model. Fertil Steril. 1991. 56:568–570.8. Kwon SW, Lim SH, Lee YW, Lee YG, Chu BY, Lee JH, et al. Anti-adhesive effect of Poloxamer/Alginate/CaCl2 mixture in the rat model. J Korean Surg Soc. 2006. 71:280–287.9. De Iaco PA, Stefanetti M, Pressato D, Piana S, Dona M, Pavesio A, et al. A novel hyaluronan-based gel in laparoscopic adhesion prevention: preclinical evaluation in an animal model. Fertil Steril. 1998. 69:318–323.10. Cainzos M, Rodriguez-Segade F, Martinez-Castro J, Prieto D, Becker MR, Aneiros F, et al. Intra-abdominal adhesions after open and laparoscopic cholecystectomy: an experimental model. J Laparoendosc Adv Surg Tech A. 2006. 16:108–112.11. Szabo G, Miko I, Peto K, Brath E, Nagy P, Gamal EM. Laparoscopic versus open cholecystectomy: reaction in the liver bed. Magy Seb. 2005. 58:106–110.12. Tsao KJ, St Peter SD, Valusek PA, Keckler SJ, Sharp S, Holcomb GW 3rd, et al. Adhesive small bowel obstruction after appendectomy in children: comparison between the laparoscopic and open approach. J Pediatr Surg. 2007. 42:939–942.13. Rosin D, Zmora O, Hoffman A, Khaikin M, Bar Zakai B, Munz Y, et al. Low incidence of adhesion-related bowel obstruction after laparoscopic colorectal surgery. J Laparoendosc Adv Surg Tech A. 2007. 17:604–607.14. Gutt CN, Oniu T, Schemmer P, Mehrabi A, Buchler MW. Fewer adhesions induced by laparoscopic surgery? Surg Endosc. 2004. 18:898–906.15. Takeuchi H, Kitade M, Kikuchi I, Shimanuki H, Kumakiri J, Takeda S. Influencing factors of adhesion development and the efficacy of adhesion-preventing agents in patients undergoing laparoscopic myomectomy as evaluated by a second-look laparoscopy. Fertil Steril. 2008. 89:1247–1253.16. Harris ES, Morgan RF, Rodeheaver GT. Analysis of the kinetics of peritoneal adhesion formation in the rat and evaluation of potential antiadhesive agents. Surgery. 1995. 117:663–669.17. Zong X, Li S, Chen E, Garlick B, Kim KS, Fang D, et al. Prevention of postsurgery-induced abdominal adhesions by electrospun bioabsorbable nanofibrous poly(lactide-co-glycolide)-based membranes. Ann Surg. 2004. 240:910–915.18. Kim K, Luu YK, Chang C, Fang D, Hsiao BS, Chu B, et al. Incorporation and controlled release of a hydrophilic antibiotic using poly(lactide-co-glycolide)-based electrospun nanofibrous scaffolds. J Control Release. 2004. 98:47–56.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Efficacy and safety of hyaluronate membrane in the rabbit cecum-abdominal wall adhesion model
- Adhesion formation after applying adhesion barrier in laparoscopic gynecologic surgery: Experience of 7 patients
- Osteogenic Nanofibrous Coated Titanium Implant Results in Enhanced Osseointegration: In Vivo Preliminary Study in a Rabbit Model
- Prevention of Adhesion Syndrome after Extraocular Muscle Surgery in Rabbit
- Effects of a Temperature-Sensitive, Anti-Adhesive Agent on the Reduction of Adhesion in a Rabbit Laminectomy Model