J Korean Soc Transplant.
2014 Mar;28(1):5-12. 10.4285/jkstn.2014.28.1.5.
Current Issues in ABO-Incompatible Kidney Transplantation
- Affiliations
-
- 1Department of Internal Medicine, The Catholic University of Korea, College of Medicine, Seoul, Korea. yangch@catholic.ac.kr
- KMID: 2202495
- DOI: http://doi.org/10.4285/jkstn.2014.28.1.5
Abstract
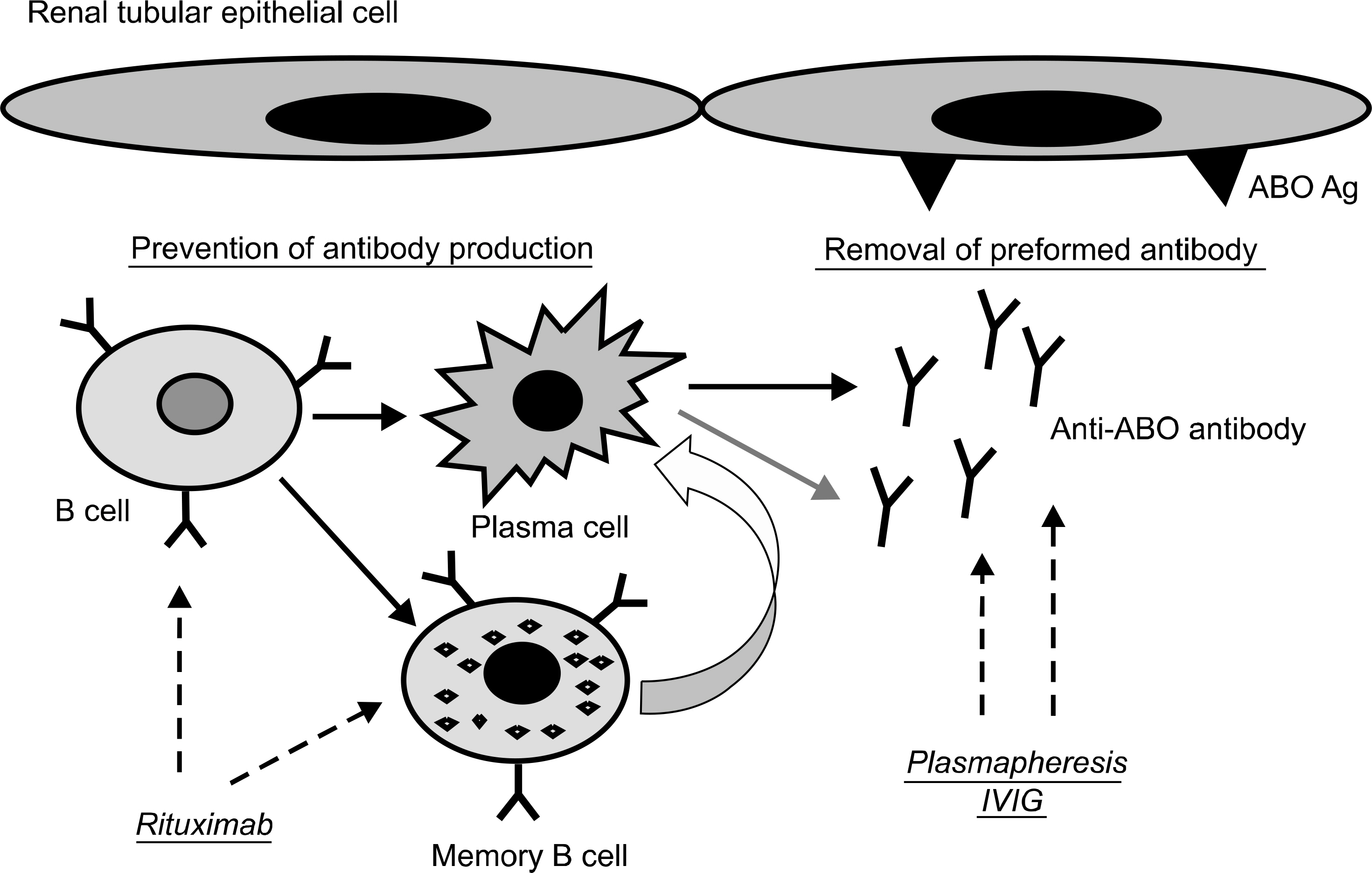
- Organ shortage is a critical issue in Korea as well as in other countries. In Korea, in 2013, the number of end-stage renal disease patients on the waiting list was 14,600; however, only 1,759 patients received transplantation during 2013. Recent advances in immunosuppression and antibody removal protocols have made ABO-incompatible kidney transplantation (ABO IKT) feasible, and have increased the opportunities for patients to undergo transplantation, especially for patients who do not have an ABO-compatible donor. The first ABO IKT was reported in 1955, but was unsuccessful due to the absence of an effective preparation protocol for antibody removal. In the 1980s, Alexandre used a protocol for removal of anti-ABO antibodies for the first time; however, the outcome was still inferior to that of ABO-compatible KT. Since 2000, with the advancement of immunosuppression and plasmapheresis, the outcome of ABO IKT has shown significant improvement and is now comparable to that of ABO-compatible KT. However, there are still several undetermined issues in ABO IKT. For example, issues regarding anti-ABO antibody titer, pretransplant desensitization method, immune suppressant regimen, and the role of C4d have still not been established. In this article, we reviewed the current status and protocol of ABO IKT and addressed to the undetermined issues in this field.
MeSH Terms
Figure
Reference
-
References
1). Korean Network for Organ Sharing (KONOS). 2013 Annual Data Report [Internet]. Seoul: KONOS;2014. [cited 2014 Jan 13]. Available from:. http://www.konos.go.kr.2). Jin DC, Ha IS, Kim NH, Lee SW, Lee JS, Yoon SR, et al. Brief report: renal replacement therapy in Korea, 2010. Kidney Res Clin Pract. 2012; 31:62–71.
Article3). Ichimaru N, Takahara S. Japan's experience with living-donor kidney transplantation across ABO barriers. Nat Clin Pract Nephrol. 2008; 4:682–92.
Article4). Tydén G, Donauer J, Wadström J, Kumlien G, Wilpert J, Nilsson T, et al. Implementation of a protocol for ABO-incompatible kidney transplantation: a three-center experience with 60 consecutive transplantations. Transplantation. 2007; 83:1153–5.5). Takahashi K, Saito K, Takahara S, Okuyama A, Tanabe K, Toma H, et al. Excellent longterm outcome of ABO-incompatible living donor kidney transplantation in Japan. Am J Transplant. 2004; 4:1089–96.
Article6). Tydén G, Kumlien G, Genberg H, Sandberg J, Lundgren T, Fehrman I. ABO incompatible kidney transplantations without splenectomy, using antigen-specific immunoadsorption and rituximab. Am J Transplant. 2005; 5:145–8.
Article7). Gloor JM, Stegall MD. ABO incompatible kidney transplantation. Curr Opin Nephrol Hypertens. 2007; 16:529–34.
Article8). Stegall MD, Dean PG, Gloor JM. ABO-incompatible kidney transplantation. Transplantation. 2004; 78:635–40.
Article9). Tobian AA, Shirey RS, Montgomery RA, Tisch DJ, Ness PM, King KE. Therapeutic plasma exchange reduces ABO titers to permit ABO-incompatible renal transplantation. Transfusion. 2009; 49:1248–54.
Article10). Montgomery JR, Berger JC, Warren DS, James NT, Montgomery RA, Segev DL. Outcomes of ABO-incompatible kidney transplantation in the United States. Transplantation. 2012; 93:603–9.
Article11). Hume DM, Merrill JP, Miller BF, Thorn GW. Experiences with renal homotransplantation in the human: report of nine cases. J Clin Invest. 1955; 34:327–82.12). Alexandre GP, Squifflet JP, De Bruyère M, Latinne D, Reding R, Gianello P, et al. Present experiences in a series of 26 ABO-incompatible living donor renal allografts. Transplant Proc. 1987; 19:4538–42.13). Reding R, Squifflet JP, Pirson Y, Jamart J, De Bruyère M, Moriau M, et al. Living-related and unrelated donor kidney transplantation: comparison between ABO-com-patible and incompatible grafts. Transplant Proc. 1987; 19(1 Pt 2):1511–3.14). Takahashi K, Yagisawa T, Sonda K, Kawaguchi H, Yamaguchi Y, Toma H, et al. ABO-incompatible kidney transplantation in a singlecenter trial. Transplant Proc. 1993; 25(1 Pt 1):271–3.15). Gloor JM, Lager DJ, Moore SB, Pineda AA, Fidler ME, Larson TS, et al. ABO-incompatible kidney transplantation using both A2 and non-A2 living donors. Transplantation. 2003; 75:971–7.
Article16). Kong JM, Lee DR, Jeong JH, Choi JH, Lee JO, Lee WR, et al. ABO blood group incompatible living donor kidney transplantation without splenectomy. J Korean Soc Transplant. 2009; 23:71–6. (공진민, 이동렬, 정준헌, 최재호, 이정호, 이화림, 등. 비장적출을 하지 않은 ABO 혈액형 부적합 생체 신장이식 경험. 대한이식학회지 2009;23: 71–6.).17). Kong JM, Ahn J, Park JB, Chung BH, Yang J, Kim JK, et al. ABO incompatible living donor kidney transplantation in Korea: highly uniform protocols and good medium-term outcome. Clin Transplant. 2013; 27:875–81.
Article18). Breimer ME, Molne J, Nordén G, Rydberg L, Thiel G, Svalander CT. Blood group A and B antigen expression in human kidneys correlated to A1/A2/B, Lewis, and se-cretor status. Transplantation. 2006; 82:479–85.
Article19). Takahashi K, Saito K. ABO-incompatible kidney transplantation. Transplant Rev (Orlando). 2013; 27:1–8.
Article20). Genberg H, Kumlien G, Wennberg L, Berg U, Tydén G. ABO-incompatible kidney transplantation using antigen-specific immunoadsorption and rituximab: a 3-year follow-up. Transplantation. 2008; 85:1745–54.
Article21). Tobian AA, Shirey RS, Montgomery RA, Ness PM, King KE. The critical role of plasmapheresis in ABO-incompa-tible renal transplantation. Transfusion. 2008; 48:2453–60.
Article22). Sonnenday CJ, Warren DS, Cooper M, Samaniego M, Haas M, King KE, et al. Plasmapheresis, CMV hyperimmune globulin, and anti-CD20 allow ABO-incompat-ible renal transplantation without splenectomy. Am J Transplant. 2004; 4:1315–22.
Article23). Chikaraishi T, Sasaki H, Tsutsumi H, Miyano S, Nakazawa R, Nakano T, et al. ABO blood type incompatible kidney transplantation without splenectomy prepared with plasma exchange and rituximab. Transplant Proc. 2008; 40:3445–7.
Article24). Vo AA, Lukovsky M, Toyoda M, Wang J, Reinsmoen NL, Lai CH, et al. Rituximab and intravenous immune globulin for desensitization during renal transplantation. N Engl J Med. 2008; 359:242–51.
Article25). Jordan SC, Toyoda M, Kahwaji J, Vo AA. Clinical aspects of intravenous immunoglobulin use in solid organ transplant recipients. Am J Transplant. 2011; 11:196–202.
Article26). Kahwaji J, Vo AA, Jordan SC. ABO blood group incompatibility: a diminishing barrier to successful kidney transplantation? Expert Rev Clin Immunol. 2010; 6:893–900.
Article27). Tanabe K, Tokumoto T, Ishida H, Ishikawa N, Miyamoto N, Kondo T, et al. Excellent outcome of ABO-incompat-ible living kidney transplantation under pretransplantation immunosuppression with tacrolimus, mycophenolate mofetil, and steroid. Transplant Proc. 2004; 36:2175–7.
Article28). Chung BH, Lee JY, Kang SH, Sun IO, Choi SR, Park HS, et al. Comparison of clinical outcome between high and low baseline anti-ABO antibody titers in ABO-incompatible kidney transplantation. Ren Fail. 2011; 33:150–8.
Article29). Ishida H, Miyamoto N, Shirakawa H, Shimizu T, Tokumoto T, Ishikawa N, et al. Evaluation of immunosuppressive regimens in ABO-incompatible living kidney transplantation: single center analysis. Am J Transplant. 2007; 7:825–31.30). Shirey RS, Cai W, Montgomery RA, Chhibber V, Ness PM, King KE. Streamlining ABO antibody titrations for monitoring ABO-incompatible kidney transplants. Transfusion. 2010; 50:631–4.31). Tanabe K. Interinstitutional variation in the measurement of anti-A/B antibodies: the Japanese ABO-Incompatible Transplantation Committee survey. Transplantation. 2007; 84(12 Suppl):S13–6.
Article32). Krishnan NS, Fleetwood P, Higgins RM, Hathaway M, Zehnder D, Mitchell D, et al. Application of flow cytometry to monitor antibody levels in ABO incompatible kidney transplantation. Transplantation. 2008; 86:474–7.
Article33). Tobian AA, Shirey RS, King KE. ABO antibody titer monitoring for incompatible renal transplantation. Transfusion. 2011; 51:454–7.
Article34). Lawrence C, Galliford JW, Willicombe MK, McLean AG, Lesabe M, Rowan F, et al. Antibody removal before ABO-incompatible renal transplantation: how much plasma exchange is therapeutic? Transplantation. 2011; 92:1129–33.
Article35). Chung BH, Lim JU, Kim Y, Kim JI, Moon IS, Choi BS, et al. Impact of the baseline anti-A/B antibody titer on the clinical outcome in ABO-incompatible kidney transplantation. Nephron Clin Pract. 2013; 124:79–88.
Article36). Wilpert J, Geyer M, Teschner S, Schaefer T, Pisarski P, Schulz-Huotari C, et al. ABO-incompatible kidney transplantation-proposal of an intensified apheresis strategy for patients with high initial isoagglutinine titers. J Clin Apher. 2007; 22:314–22.
Article37). Montgomery RA. Renal transplantation across HLA and ABO antibody barriers: integrating paired donation into desensitization protocols. Am J Transplant. 2010; 10:449–57.
Article38). Uchida J, Iwai T, Kato M, Machida Y, Naganuma T, Kumada N, et al. A novel approach to successful ABO-incompatible high-titer renal transplantation. Transplant Proc. 2008; 40:2285–8.
Article39). Koshino K, Okamoto M, Sakai K, Suzuki T, Nobori S, Matsuyama M, et al. The excellent outcomes of ABO-incompatible kidney transplantation with high titer (>x2048) using anti-CD20 and anti-CD25 antibody without splenectomy: two case reports. Transplant Proc. 2011; 43:2379–82.40). Shimmura H, Tanabe K, Ishida H, Tokumoto T, Ishikawa N, Miyamoto N, et al. Lack of correlation between results of ABO-incompatible living kidney transplantation and anti-ABO blood type antibody titers under our current immunosuppression. Transplantation. 2005; 80:985–8.
Article41). Becker YT, Samaniego-Picota M, Sollinger HW. The emerging role of rituximab in organ transplantation. Transpl Int. 2006; 19:621–8.
Article42). Pescovitz MD. Rituximab, an anti-cd20 monoclonal antibody: history and mechanism of action. Am J Transplant. 2006; 6:859–66.
Article43). Kohei N, Hirai T, Omoto K, Ishida H, Tanabe K. Chronic antibody-mediated rejection is reduced by targeting B-cell immunity during an introductory period. Am J Transplant. 2012; 12:469–76.
Article44). Egawa H, Teramukai S, Haga H, Tanabe M, Mori A, Ikegami T, et al. Impact of rituximab desensitization on blood-type-incompatible adult living donor liver transplantation: a Japanese multicenter study. Am J Transplant. 2014; 14:102–14.
Article45). Fuchinoue S, Ishii Y, Sawada T, Murakami T, Iwadoh K, Sannomiya A, et al. The 5-year outcome of ABO-incompatible kidney transplantation with rituximab induction. Transplantation. 2011; 91:853–7.
Article46). Marwick C. Monoclonal antibody to treat lymphoma. JAMA. 1997; 278(616):618.
Article47). Coiffier B, Haioun C, Ketterer N, Engert A, Tilly H, Ma D, et al. Rituximab (anti-CD20 monoclonal antibody) for the treatment of patients with relapsing or refractory aggressive lymphoma: a multicenter phase II study. Blood. 1998; 92:1927–32.48). Vieira CA, Agarwal A, Book BK, Sidner RA, Bearden CM, Gebel HM, et al. Rituximab for reduction of anti-HLA antibodies in patients awaiting renal transplantation: 1. Safety, pharmacodynamics, and pharmacokinetics. Transplantation. 2004; 77:542–8.49). Toki D, Ishida H, Horita S, Setoguchi K, Yamaguchi Y, Tanabe K. Impact of low-dose rituximab on splenic B cells in ABO-incompatible renal transplant recipients. Transpl Int. 2009; 22:447–54.
Article50). Chung BH, Hong YA, Sun IO, Piao SG, Kim JI, Moon IS, et al. Determination of rituximab dose according to immunologic risk in ABO-incompatible kidney transplantation. Ren Fail. 2012; 34:974–9.
Article51). Chung BH, Yun JT, Ha SE, Kim JI, Moon IS, Choi BS, et al. Combined use of rituximab and plasmapheresis pretransplant increases post-transplant infections in renal transplant recipients with basiliximab induction therapy. Transpl Infect Dis. 2013; 15:559–68.
Article52). Habicht A, Bröker V, Blume C, Lorenzen J, Schiffer M, Richter N, et al. Increase of infectious complications in ABO-incompatible kidney transplant recipients: a single centre experience. Nephrol Dial Transplant. 2011; 26:4124–31.53). Grim SA, Pham T, Thielke J, Sankary H, Oberholzer J, Benedetti E, et al. Infectious complications associated with the use of rituximab for ABO-incompatible and positive crossmatch renal transplant recipients. Clin Transplant. 2007; 21:628–32.
Article54). Flint SM, Walker RG, Hogan C, Haeusler MN, Robertson A, Francis DM, et al. Successful ABO-incompatible kidney transplantation with antibody removal and standard immunosuppression. Am J Transplant. 2011; 11:1016–24.
Article55). Oettl T, Zuliani E, Gaspert A, Hopfer H, Dickenmann M, Fehr T. Late steroid withdrawal after ABO blood group-incompatible living donor kidney transplantation: high rate of mild cellular rejection. Transplantation. 2010; 89:702–6.
Article56). Tobian AA, Shirey RS, Montgomery RA, Cai W, Haas M, Ness PM, et al. ABO antibody titer and risk of antibody-mediated rejection in ABO-incompatible renal transplantation. Am J Transplant. 2010; 10:1247–53.
Article57). Park WD, Grande JP, Ninova D, Nath KA, Platt JL, Gloor JM, et al. Accommodation in ABO-incompatible kidney allografts, a novel mechanism of self-protection against antibody-mediated injury. Am J Transplant. 2003; 3:952–60.
Article58). Kirk AD, Baldwin WM, Cascalho MI, Chong AS, Sykes M, West LJ. American society of transplantation symposium on B cells in transplantation: harnessing humoral immunity from rodent models to clinical practice. Am J Transplant. 2007; 7:1464–70.
Article59). Lynch RJ, Platt JL. Accommodation in renal transplantation: unanswered questions. Curr Opin Organ Transplant. 2010; 15:481–5.
Article60). Aikawa A, Ohara T, Arai K, Hadano T, Kawamura T, Sugiyama K, et al. Clinical outcome and accommodation in ABO incompatible kidney transplantation. Clin Transplant. 2004; 135–42.61). Solez K, Colvin RB, Racusen LC, Haas M, Sis B, Mengel M, et al. Banff 07 classification of renal allograft pathology: updates and future directions. Am J Transplant. 2008; 8:753–60.
Article62). Haas M, Segev DL, Racusen LC, Bagnasco SM, Locke JE, Warren DS, et al. C4d deposition without rejection correlates with reduced early scarring in ABO-incompat-ible renal allografts. J Am Soc Nephrol. 2009; 20:197–204.
Article63). Setoguchi K, Ishida H, Shimmura H, Shimizu T, Shirakawa H, Omoto K, et al. Analysis of renal transplant protocol biopsies in ABO-incompatible kidney transplantation. Am J Transplant. 2008; 8:86–94.
Article64). Haas M, Sis B, Racusen LC, Solez K, Glotz D, Colvin RB, et al. Banff 2013 meeting report: inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant. 2014; 14:272–83.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- ABO-Incompatible Kidney Transplantation
- Analysis of the Results of ABO-Incompatible Kidney Transplantation: In Comparison with ABO-Compatible Kidney Transplantation
- Successful Pediatric ABO-Incompatible Kidney Transplantation without Pretransplant Plasmapheresis: Report of a Case
- Case of ABO-Incompatible Living Donor Kidney Transplantation without Blood Products in a Jehovah's Witness
- ABO-Incompatible Living Donor Liver Transplantation