J Korean Neurosurg Soc.
2012 Jul;52(1):32-36. 10.3340/jkns.2012.52.1.32.
Electrophysiological and Behavioral Changes by Phosphodiesterase 4 Inhibitor in a Rat Model of Alcoholic Neuropathy
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Yonsei University Wonju College of Medicine, Wonju, Korea.
- 2Department of Anesthesiology and Pain Medicine, Seoul National University Hospital, Seoul, Korea.
- 3Department of Rehabilitation Medicine, Wonju Christian Hospital, Yonsei University Wonju College of Medicine, Wonju, Korea.
- 4Department of Preventive Medicine and Institute of Occupational Medicine, Institute of Occupational & Environmental Medicine, Yonsei University Wonju College of Medicine, Wonju, Korea.
- 5Department of Neurosurgery, College of Medicine, Chosun University, Gwangju, Korea. chosunns@chosun.ac.kr
- KMID: 2190520
- DOI: http://doi.org/10.3340/jkns.2012.52.1.32
Abstract
OBJECTIVE
Alcoholic neuropathy is characterized by allodynia (a discomfort evoked by normally innocuous stimuli), hyperalgesia (an exaggerated pain in response to painful stimuli) and spontaneous burning pain. The aim of the present study is to investigate the effect of rolipram, a phosphodiesterase 4 inhibitor, against alcohol-induced neuropathy in rats.
METHODS
Allodynia was induced by administering 35% v/v ethanol (10 g/kg; oral gavage) to Spraue-Dawley rats for 8 weeks. Rolipram and saline (vehicle) were administered intraperitoneally. Mechanical allodynia was measured by using von Frey filaments. Somatosensory evoked potential (SEP) was proposed as complementary measure to assess the integrity of nerve pathway.
RESULTS
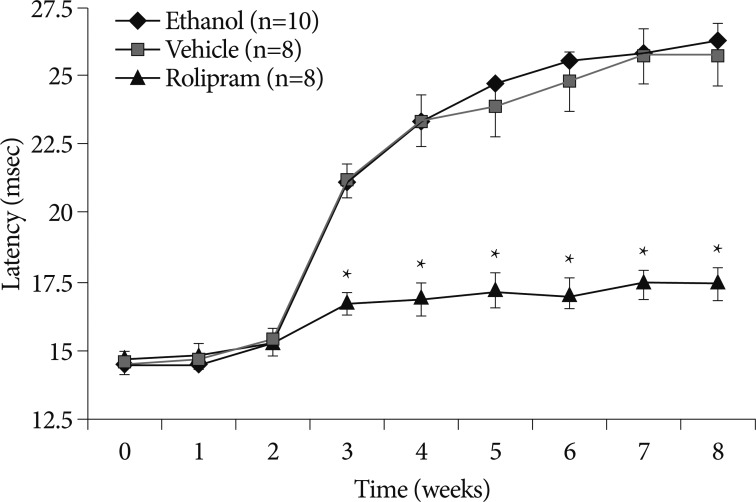
The ethanol-induced mechanical allodynia began to manifest from 3 week, and then peaked within 1 week. Beginning from 3 week, latency significantly started to increased in control group. In rolipram treated rats, the shorter latency was sustained until 8 weeks (p<0.05). The mechanical allodynia, which began to manifest on the 3 weeks, intraperitoneal injections of rolipram sustained statistical difference until 8 weeks, the final week of the study (p<0.05).
CONCLUSION
This study suggests that rolipram might alleviate mechanical allodynia induced by alcohol in rats, which clearly has clinical implication.
MeSH Terms
Figure
Reference
-
1. Atkins CM, Oliva AA Jr, Alonso OF, Pearse DD, Bramlett HM, Dietrich WD. Modulation of the cAMP signaling pathway after traumatic brain injury. Exp Neurol. 2007; 208:145–158. PMID: 17916353.
Article2. Bosch EP, Pelham RW, Rasool CG, Chatterjee A, Lash RW, Brown L, et al. Animal models of alcoholic neuropathy : morphologic, electrophysiologic, and biochemical findings. Muscle Nerve. 1979; 2:133–144. PMID: 232540.
Article3. Brain WR, Walton JN. Disorders of peripheral nerves : alcoholoc polyneuritis. Brain's deseases of the nervous system. 1969. 7th ed. London: Oxford;p. 817–819.4. Cogswell JP, Godlevski MM, Wisely GB, Clay WC, Leesnitzer LM, Ways JP, et al. NF-kappa B regulates IL-1 beta transcription through a consensus NF-kappa B binding site and a nonconsensus CRE-like site. J Immunol. 1994; 153:712–723. PMID: 8021507.5. Corsetti G, Rezzani R, Rodella L, Bianchi R. Ultrastructural study of the alterations in spinal ganglion cells of rats chronically fed on ethanol. Ultrastruct Pathol. 1998; 22:309–319. PMID: 9805356.
Article6. Dina OA, Barletta J, Chen X, Mutero A, Martin A, Messing RO, et al. Key role for the epsilon isoform of protein kinase C in painful alcoholic neuropathy in the rat. J Neurosci. 2000; 20:8614–8619. PMID: 11069970.
Article7. Dixon WJ. Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol. 1980; 20:441–462. PMID: 7387124.
Article8. Fillmore KM. Women's drinking across the adult life course as compared to men's. Br J Addict. 1987; 82:801–811. PMID: 3478069.
Article9. Floyd RA. Antioxidants, oxidative stress, and degenerative neurological disorders. Proc Soc Exp Biol Med. 1999; 222:236–245. PMID: 10601882.10. Floyd RA, Hensley K. Oxidative stress in brain aging. Implications for therapeutics of neurodegenerative diseases. Neurobiol Aging. 2002; 23:795–807. PMID: 12392783.
Article11. Gomberg ES. Women and alcohol : use and abuse. J Nerv Ment Dis. 1993; 181:211–219. PMID: 8473872.12. Hou S, Guan H, Ricciardi RP. Phosphorylation of serine 337 of NF-kappaB p50 is critical for DNA binding. J Biol Chem. 2003; 278:45994–45998. PMID: 12947093.13. Hu S, Peterson PK, Chao CC. Cytokine-mediated neuronal apoptosis. Neurochem Int. 1997; 30:427–431. PMID: 9106257.
Article14. Keeling KL, Hicks RR, Mahesh J, Billings BB, Kotwal GJ. Local neutrophil influx following lateral fluid-percussion brain injury in rats is associated with accumulation of complement activation fragments of the third component (C3) of the complement system. J Neuroimmunol. 2000; 105:20–30. PMID: 10713360.
Article15. Kinoshita K, Chatzipanteli K, Vitarbo E, Truettner JS, Alonso OF, Dietrich WD. Interleukin-1beta messenger ribonucleic acid and protein levels after fluid-percussion brain injury in rats : importance of injury severity and brain temperature. Neurosurgery. 2002; 51:195–203. discussion 2003. PMID: 12182417.
Article16. Koike H, Iijima M, Sugiura M, Mori K, Hattori N, Ito H, et al. Alcoholic neuropathy is clinicopathologically distinct from thiamine-deficiency neuropathy. Ann Neurol. 2003; 54:19–29. PMID: 12838517.
Article17. Lander HM. An essential role for free radicals and derived species in signal transduction. FASEB J. 1997; 11:118–124. PMID: 9039953.
Article18. Manganiello VC, Murata T, Taira M, Belfrage P, Degerman E. Diversity in cyclic nucleotide phosphodiesterase isoenzyme families. Arch Biochem Biophys. 1995; 322:1–13. PMID: 7574662.
Article19. Mayr B, Montminy M. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat Rev Mol Cell Biol. 2001; 2:599–609. PMID: 11483993.
Article20. Meda L, Cassatella MA, Szendrei GI, Otvos L Jr, Baron P, Villalba M, et al. Activation of microglial cells by beta-amyloid protein and interferon-gamma. Nature. 1995; 374:647–650. PMID: 7715705.
Article21. Monforte R, Estruch R, Valls-Solé J, Nicolás J, Villalta J, Urbano-Marquez A. Autonomic and peripheral neuropathies in patients with chronic alcoholism. A dose-related toxic effect of alcohol. Arch Neurol. 1995; 52:45–51. PMID: 7826275.
Article22. Naik AK, Tandan SK, Dudhgaonkar SP, Jadhav SH, Kataria M, Prakash VR, et al. Role of oxidative stress in pathophysiology of peripheral neuropathy and modulation by N-acetyl-L-cysteine in rats. Eur J Pain. 2006; 10:573–579. PMID: 16214382.
Article23. Narita M, Miyoshi K, Narita M, Suzuki T. Involvement of microglia in the ethanol-induced neuropathic pain-like state in the rat. Neurosci Lett. 2007; 414:21–25. PMID: 17284346.
Article24. Nikulina E, Tidwell JL, Dai HN, Bregman BS, Filbin MT. The phosphodiesterase inhibitor rolipram delivered after a spinal cord lesion promotes axonal regeneration and functional recovery. Proc Natl Acad Sci U S A. 2004; 101:8786–8790. PMID: 15173585.
Article25. Pearse DD, Pereira FC, Marcillo AE, Bates ML, Berrocal YA, Filbin MT, et al. cAMP and Schwann cells promote axonal growth and functional recovery after spinal cord injury. Nat Med. 2004; 10:610–616. PMID: 15156204.
Article26. Pentney RJ, Quackenbush LJ. Dendritic hypertrophy in Purkinje neurons of old Fischer 344 rats after long-term ethanol treatment. Alcohol Clin Exp Res. 1990; 14:878–886. PMID: 2088124.
Article27. Schaal SM, Golshani R, Ghosh M, Lovera L, Lopez M, Patel M, et al. Targeting phosphodiesterase-4 after spinal cord injury using pharmacological and molecular approches. J Neurotrauma. 2008; 25:297.28. Sharma SS, Sayyed SG. Effects of trolox on nerve dysfunction, thermal hyperalgesia and oxidative stress in experimental diabetic neuropathy. Clin Exp Pharmacol Physiol. 2006; 33:1022–1028. PMID: 17042909.
Article29. Soares HD, Hicks RR, Smith D, McIntosh TK. Inflammatory leukocytic recruitment and diffuse neuronal degeneration are separate pathological processes resulting from traumatic brain injury. J Neurosci. 1995; 15:8223–8233. PMID: 8613756.
Article30. Tanaka M, Sotomatsu A, Yoshida T, Hirai S, Nishida A. Detection of superoxide production by activated microglia using a sensitive and specific chemiluminescence assay and microglia-mediated PC12h cell death. J Neurochem. 1994; 63:266–270. PMID: 8207432.
Article31. Verghese MW, McConnell RT, Strickland AB, Gooding RC, Stimpson SA, Yarnall DP, et al. Differential regulation of human monocyte-derived TNF alpha and IL-1 beta by type IV cAMP-phosphodiesterase (cAMP-PDE) inhibitors. J Pharmacol Exp Ther. 1995; 272:1313–1320. PMID: 7891349.32. Victor M. Dyke PJ, Thomas PK, Lambert EH, editors. Polyneuropathy due to nutritional deficiency and alcoholism. Peripheral neuropathy. 1975. Philadelphia: WB Saunders Co;p. 1030–1066.33. Vitarbo EA, Chatzipanteli K, Kinoshita K, Truettner JS, Alonso OF, Dietrich WD. Tumor necrosis factor alpha expression and protein levels after fluid percussion injury in rats : the effect of injury severity and brain temperature. Neurosurgery. 2004; 55:416–424. discussion 424-425. PMID: 15271250.34. Yerdelen D, Koc F, Uysal H. Strength-duration properties of sensory and motor axons in alcoholic polyneuropathy. Neurol Res. 2008; 30:746–750. PMID: 18489821.
Article35. Zou J, Rabin RA, Pentney RJ. Ethanol enhances neurite outgrowth in primary cultures of rat cerebellar macroneurons. Brain Res Dev Brain Res. 1993; 72:75–84.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical and Electrophysiological Characteristics of Alcoholic Neuropathy
- The Effect of Phosphodiesterase-4-Specific Inhibitor in the Rat Model of Spinal Nerve Ligation
- Effects of Mirodenafil, a Phosphodiesterase-5 Inhibitor, on Female Rat Bladder in a Partial Bladder Outlet Obstruction Model: Physiological and Immunohistochemical Aspects
- Effects of Chronic Treatment with a Type 5 Phosphodiesterase Inhibitor on Erectile Function in Diabetic Rats
- Potential of histone deacetylase 6 inhibitors in alleviating chemotherapy-induced peripheral neuropathy