J Adv Prosthodont.
2009 Mar;1(1):47-55. 10.4047/jap.2009.1.1.47.
Comparion of stability in titanium implants with different surface topographies in dogs
- Affiliations
-
- 1Department of Prosthodontics, Graduate School, Chonnam National University, Gwang-Ju, Korea. yhsdent@chonnam.ac.kr
- KMID: 2176344
- DOI: http://doi.org/10.4047/jap.2009.1.1.47
Abstract
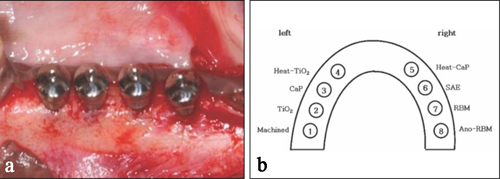
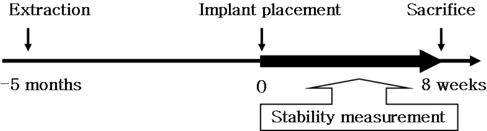
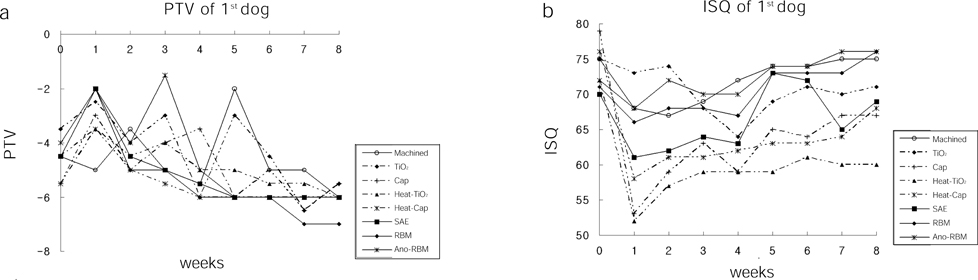
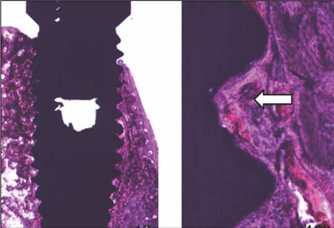
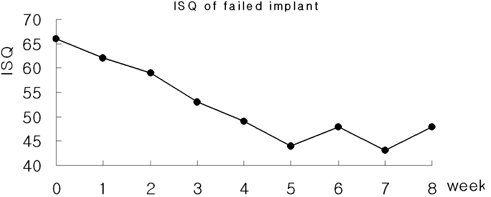
- STATEMENT OF PROBLEM: A few of studies which compared and continuously measured the stability of various surface treated implants in the same individual had been performed. PURPOSE: We aim to find the clinical significance of surface treatments by observing the differences in the stabilization stages of implant stability. MATERIAL AND METHODS: Eight different surface topographies of dental implants were especially designed for the present study. Machined surface implants were used as a control group. 4 nano-treated surface implants (20 nm TiO2 coating surface, heat-treated 80 nm TiO2 coating surface, CaP coating surface, heat treated CaP coating surface) and 3 micro-treated surface implants [resorbable blast media (RBM) surface, sandblast and acid-etched (SAE) surface, anodized RBM surface] were used as experiment groups. All 24 implants were placed in 3 adult dogs. Periotest(R) & ISQ values measured for 8 weeks and all animals were sacrificed at 8 weeks after surgery. Then the histological analyses were done. RESULTS: In PTV, all implants were stabilized except 1 failed implants. In ISQ values, The lowest stability was observed at different times for each individual. The ISQ values were showed increased tendency after 5 weeks in every groups. After 4 to 5 weeks, the values were stabilized. There was no statistical correlation between the ISQ values and PTV. In the histological findings, the bone formation was observed to be adequate in general and no differences among the 8 surface treated implants. CONCLUSIONS: In this study, the difference in the stability of the implants was determined not by the differences in the surface treatment but by the individual specificity.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
The effects of local factors on the survival of dental implants: A 19 year retrospective study
Sung Hoi Kim, Sunjai Kim, Keun-Woo Lee, Dong-Hoo Han
J Korean Acad Prosthodont. 2010;48(1):28-40. doi: 10.4047/jkap.2010.48.1.28.
Reference
-
1. Albrektsson T, Lekholm U. Osseointegration: current state of the art. Dent Clin North Am. 1989. 33:537–554.2. Ericsson I, Johansson CB, Bystedt H, Norton MR. A histomorphometric evaluation of bone-to-implant contact on machine-prepared and roughened titanium dental implants. A pilot study in the dog. Clin Oral Implants Res. 1994. 5:202–206.3. Schatzker J, Horne JG, Sumner-Smith G. The effect of movement on the holding power of screws in bone. Clin Orthop Relat Res. 1975. 111:257–262.4. Huang HM, Chiu CL, Yeh CY, Lin CT, Lin LH, Lee SY. Early detection of implant healing process using resonance frequency analysis. Clin Oral Implants Res. 2003. 14:437–443.5. Parel SM, Triplett RG. Immediate fixture placement: a treatment planning alternative. Int J Oral Maxillofac Implants. 1990. 5:337–345.6. Meredith N, Friberg B, Sennerby L, Aparicio C. Relationship between contact time measurements and PTV values when using the Periotest to measure implant stability. Int J Prosthodont. 1998. 11:269–275.7. Meredith N. Assessment of implant stability as a prognostic determinant. Int J Prosthodont. 1998. 11:491–501.8. Meredith N, Book K, Friberg B, Jemt T, Sennerby L. Resonance frequency measurements of implant stability in vivo. A cross-sectional and longitudinal study of resonance frequency measurements on implants in the edentulous and partially dentate maxilla. Clin Oral Implants Res. 1997. 8:226–233.9. Meredith N, Shagaldi F, Alleyne D, Sennerby L, Cawley P. The application of resonance frequency measurements to study the stability of titanium implants during healing in the rabbit tibia. Clin Oral Implants Res. 1997. 8:234–243.10. Sennerby L, Roos J. Surgical determinants of clinical success of osseointegrated oral implants: a review of the literature. Int J Prosthodont. 1998. 11:408–420.11. Sennerby L, Meredith N. Resonance frequency analysis: measuring implant stability and osseointegration. Compend Contin Educ Dent. 1998. 19:493–498. 500502quiz 504.12. Park CJ. A study on the change of implant stability using resonance frequency analysis. SNUDH Dept of Pros. Unpublished 2003.13. Nedir R, Bischof M, Szmukler-Moncler S, Bernard JP, Samson J. Predicting osseointegration by means of implant primary stability. Clin Oral Implants Res. 2004. 15:520–528.14. Berglundh T, Abrahamsson I, Lang NP, Lindhe J. DDe novo alveolar bone formation adjacent to endosseous implants. Clin Oral Implants Res. 2003. 14:251–262.15. Misch CE. Density of bone: effect on treatment plans, surgical approach, healing, and progressive boen loading. Int J Oral Implantol. 1990. 6:23–31.16. Albrektsson T, Jacobsson M. Bone-metal interface in osseointegration. J Prosthet Dent. 1987. 57:597–607.17. Collier JP, Mayor MB, Chae JC, Surprenant VA, Surprenant HP, Dauphinais LA. Macroscopic and microscopic evidence of prosthetic fixation with porous-coated materials. Clin Orthop Relat Res. 1988. 235:173–180.18. Pilliar RM. Porous-surfaced metallic implants for orthopedic applications. J Biomed Mater Res. 1987. 21:1–33.19. Sun L, Berndt CC, Gross KA, Kucuk A. Material fundamentals and clinical performance of plasma-sprayed hydroxyapatite coatings: a review. J Biomed Mater Res. 2001. 58:570–592.20. Tal H, Dayan D. Spontaneous early exposure of submerged implants: II. Histopathology and histomorphometry of non-perforated mucosa covering submerged implants. J Periodontol. 2000. 71:1224–1230.21. Uchida M, Kim HM, Kokubo T, Fujibayashi S, Nakamura T. Structural dependence of apatite formation on titania gels in a simulated body fluid. J Biomed Mater Res A. 2003. 64:164–170.22. Ducheyne P, Van Raemdonck W, Heughebaert JC, Heughebaert M. Structural analysis of hydroxyapatite coatings on titanium. Biomaterials. 1986. 7:97–103.23. Cooley DR, Van Dellen AF, Burgess JO, Windeler AS. The advantages of coated titanium implants prepared by radiofrequency sputtering from hydroxyapatite. J Prosthet Dent. 1992. 67:93–100.24. De Andrade MC, Sader MS, Filgueiras MR, Ogasawara T. Microstructure of ceramic coating on titanium surface as a result of hydrothermal treatment. J Mater Sci Mater Med. 2000. 11:751–755.25. Kim HM, Miyaji F, Kokubo T, Nakamura T. Preparation of bioactive Ti and its alloys via simple chemical surface treatment. J Biomed Mater Res. 1996. 32:409–417.26. Webster TJ, Ergun C, Doremus RH, Siegel RW, Bizios R. Enhanced functions of osteoblasts on nanophase ceramics. Biomaterials. 2000. 21:1803–1810.27. Webster TJ, Schadler LS, Siegel RW, Bizios R. Mechanisms of enhanced osteoblast adhesion on nanophase alumina involve vitronectin. Tissue Eng. 2001. 7:291–301.28. Oh SH, Finõnes RR, Daraio C, Chen LH, Jin S. Growth of nanoscale hydroxyapatite using chemically treated titanium oxide nanotubes. Biomaterials. 2005. 26:4938–4943.29. Karlsson M, Pålsgård E, Wilshaw PR, Di Silvio L. Initial in vitro interaction of osteoblasts with nano-porous alumina. Biomaterials. 2003. 24:3039–3046.30. Derhami K, Wolfaardt JF, Faulkner G, Grace M. Assessment of the periotest device in baseline mobility measurements of craniofacial implants. Int J Oral Maxillofac Implants. 1995. 10:221–229.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Bone Healing around Screw-shaped Titanium Implants with Three Different Surface Topographies
- Histomorphometry and stability analysis of early loaded implants with two different surface conditions in beagle dogs
- Comparision of Osseointegration Depending on Surface Treatment
- Teh Effect of Hydroxyapatite Coating on the Mechanical Strengths and Histologic Profiles of Porous Titanium Implants in Dogs
- Histologic evaluation and removal torque analysis of nano- and microtreated titanium implants in the dogs